Thiol peroxidases ameliorate LRRK2 mutant-induced mitochondrial and dopaminergic neuronal degeneration in Drosophila
- PMID: 24459295
- PMCID: PMC4030771
- DOI: 10.1093/hmg/ddu026
Thiol peroxidases ameliorate LRRK2 mutant-induced mitochondrial and dopaminergic neuronal degeneration in Drosophila
Abstract
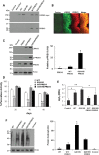
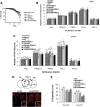
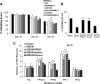
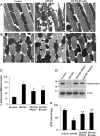
Mutations in leucine-rich repeat kinase 2 (LRRK2) are common causes of familial Parkinson's disease (PD). LRRK2 has been shown to bind peroxiredoxin-3 (PRDX3), the most important scavenger of hydrogen peroxide in the mitochondria, in vitro. Here, we examined the interactions of LRRK2 and PRDX3 in Drosophila models by crossing transgenic LRRK2 and PRDX3 flies. As proof of principle experiments, we subsequently challenged LRRK2 and LRRK2/PRDX3 flies with a peroxidase mimic, Ebselen. We demonstrated that co-expression of PRDX3 with the LRRK2 kinase mutant G2019S in bigenic Drosophila ameliorated the G2019S mutant-induced reduction in peroxidase capacity, loss of dopaminergic neurons, shortened lifespan and mitochondrial defects of flight muscles in monogenic flies expressing the G2019S alone. Challenges with Ebselen recapitulated similar rescue of these phenotypic features in mutant-expressing Drosophila. The peroxidase mimic preserved neuronal and mitochondrial and neuronal integrity and improved mobility and survival in mutant-expressing Drosophila. Taken together, our study provides the first in vivo evidence to suggest that phosphoinhibition of endogenous peroxidases could be a mechanism in LRRK2-induced oxidant-mediated neurotoxicity. Our therapeutic experiments also highlight the potential of thiol peroxidases as neuroprotective agents in PD patients carrying LRRK2 mutations.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B., et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. - PubMed
-
- Hernandez D.G., Paisán-Ruíz C., McInerney-Leo A., Jain S., Meyer-Lindenberg A., Evans E.W., Berman K.F., Johnson J., Auburger G., Schäffer A.A., et al. Clinical and positron emission tomography of Parkinson's disease caused by LRRK2. Ann. Neurol. 2005;57:453–456. - PubMed
-
- Paisan-Ruiz C., Jain S., Evans E.W., Gilks W.P., Simon J., van der Brug M., Lopez de Munain A., Aparicio S., Gil A.M., Khan N., et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. - PubMed
-
- Kachergus J., Mata I.F., Hulihan M., Taylor J.P., Lincoln S., Aasly J., Gibson J.M., Ross O.A., Lynch T., Wiley J., et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am. J. Hum. Genet. 2005;76:672–680. - PMC - PubMed
-
- Di Fonzo A., Tassorelli C., De Mari M., Chien H.F., Ferreira J., Rohe C.F., Riboldazzi G., Antonini A., Albani G., Mauro A., et al. Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson's disease. Eur. J. Hum. Genet. 2006;14:322–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

