Equilibrium and dynamic osmotic behaviour of aqueous solutions with varied concentration at constant and variable volume
- PMID: 24459448
- PMCID: PMC3888744
- DOI: 10.1155/2013/876897
Equilibrium and dynamic osmotic behaviour of aqueous solutions with varied concentration at constant and variable volume
Abstract
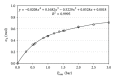
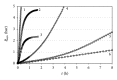
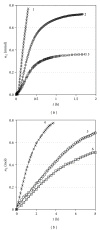
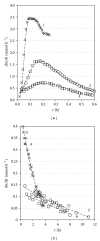
Osmosis is essential for the living organisms. In biological systems the process usually occurs in confined volumes and may express specific features. The osmotic pressure in aqueous solutions was studied here experimentally as a function of solute concentration (0.05-0.5 M) in two different regimes: of constant and variable solution volume. Sucrose, a biologically active substance, was chosen as a reference solute for the complex tests. A custom made osmotic cell was used. A novel operative experimental approach, employing limited variation of the solution volume, was developed and applied for the purpose. The established equilibrium values of the osmotic pressure are in agreement with the theoretical expectations and do not exhibit any evident differences for both regimes. In contrast, the obtained kinetic dependences reveal striking divergence in the rates of the process at constant and varied solution volume for the respective solute concentrations. The rise of pressure is much faster at constant solution volume, while the solvent influx is many times greater in the regime of variable volume. The results obtained suggest a feasible mechanism for the way in which the living cells rapidly achieve osmotic equilibrium upon changes in the environment.
Figures


Similar articles
-
Osmotic potential calculations of inorganic and organic aqueous solutions over wide solute concentration levels and temperatures.Med Phys. 2016 Jan;43(1):225. doi: 10.1118/1.4938263. Med Phys. 2016. PMID: 26745915
-
Osmosis and solute-solvent drag: fluid transport and fluid exchange in animals and plants.Cell Biochem Biophys. 2005;42(3):277-345. doi: 10.1385/CBB:42:3:277. Cell Biochem Biophys. 2005. PMID: 15976460 Review.
-
Osmotic, diffusive and convective volume and solute flows of ionic solutions through a horizontally mounted polymeric membrane.Polim Med. 2007;37(3):31-46. Polim Med. 2007. PMID: 18251203
-
Kinetic model of osmosis through semipermeable and solute-permeable membranes.Acta Physiol Scand. 2003 Feb;177(2):107-17. doi: 10.1046/j.1365-201X.2003.01062.x. Acta Physiol Scand. 2003. PMID: 12558549
-
Evolving ideas about osmosis and capillary fluid exchange.FASEB J. 1999 Feb;13(2):213-31. doi: 10.1096/fasebj.13.2.213. FASEB J. 1999. PMID: 9973310 Review.
Cited by
-
Acid-base implications of the Gibbs-Donnan effect during continuous veno-venous hemofiltration.J Nephrol. 2025 Apr;38(3):1025-1034. doi: 10.1007/s40620-025-02238-0. Epub 2025 Mar 10. J Nephrol. 2025. PMID: 40059273 Free PMC article.
-
Experimental Estimation of Membrane Tension Induced by Osmotic Pressure.Biophys J. 2016 Nov 15;111(10):2190-2201. doi: 10.1016/j.bpj.2016.09.043. Biophys J. 2016. PMID: 27851942 Free PMC article.
References
-
- van’t Hoff JH. Die Rolle des osmotischen Druckes in Analogie zwischen Lösungen und Gasen. Zeitschrift für Physikalische Chemie. 1887;1:481–493.
-
- Morse HN, Frazer JCW, Rogers FM. The osmotic pressure of glucose solutions in the vicinity of the freezing point of water. Journal of the American Chemical Society. 1907;38:175–181.
-
- Frazer JCW, Myrick RT. The osmotic pressure of sucrose solutions at 30°C. Journal of the American Chemical Society. 1916;38(10):1921–1922.
-
- Lotz P, Frazer JCW. The osmotic pressures of concentrated solutions of sucrose as determined by the water interferometer. Journal of the American Chemical Society. 1921;43(12):2501–2507.
-
- Stigter D. Interactions in aqueous solutions. II. Osmotic pressure and osmotic coefficient of sucrose and glucose solutions. Journal of Physical Chemistry. 1960;64(1):118–124.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

