Patterning expression of regenerative growth factors using high intensity focused ultrasound
- PMID: 24460731
- PMCID: PMC4186636
- DOI: 10.1089/ten.TEC.2013.0518
Patterning expression of regenerative growth factors using high intensity focused ultrasound
Abstract
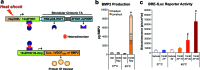
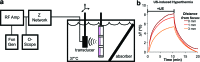
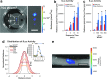
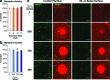
Temporal and spatial control of growth factor gradients is critical for tissue patterning and differentiation. Reinitiation of this developmental program is also required for regeneration of tissues during wound healing and tissue regeneration. Devising methods for reconstituting growth factor gradients remains a central challenge in regenerative medicine. In the current study we develop a novel gene therapy approach for temporal and spatial control of two important growth factors in bone regeneration, vascular endothelial growth factor, and bone morphogenetic protein 2, which involves application of high intensity focused ultrasound to cells engineered with a heat-activated- and ligand-inducible gene switch. Induction of transgene expression was tightly localized within cell-scaffold constructs to subvolumes of ∼30 mm³, and the amplitude and projected area of transgene expression was tuned by the intensity and duration of ultrasound exposure. Conditions for ultrasound-activated transgene expression resulted in minimal cytotoxicity and scaffold damage. Localized regions of growth factor expression also established gradients in signaling activity, suggesting that patterns of growth factor expression generated by this method will have utility in basic and applied studies on tissue development and regeneration.
Figures

References
-
- Maes C., and Cermeliet G.Vascular and nonvascular roles of VEGF in bone development. In: Ruhrberg C., ed. VEGF in Development; Springer, Austin, pp. 79–90, 2008
-
- Bénazet J-D., Bischofberger M., Tiecke E., Gonçalves A., Martin J.F., Zuniga A., et al. . A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Science 323,1050, 2009 - PubMed
-
- Ferguson C., Alpern E., Miclau T., and Helms J.Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev 87,57, 1999 - PubMed
-
- Gerstenfeld L.C., Cullinane D.M., Barnes G.L., Graves D.T., and Einhorn T.A.Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 88,873, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

