Effectiveness and cost-effectiveness of a telehealth intervention to support the management of long-term conditions: study protocol for two linked randomized controlled trials
- PMID: 24460845
- PMCID: PMC3906859
- DOI: 10.1186/1745-6215-15-36
Effectiveness and cost-effectiveness of a telehealth intervention to support the management of long-term conditions: study protocol for two linked randomized controlled trials
Abstract
Background: As the population ages, more people are suffering from long-term health conditions (LTCs). Health services around the world are exploring new ways of supporting people with LTCs and there is great interest in the use of telehealth: technologies such as the Internet, telephone and home self-monitoring.
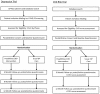
Methods/design: This study aims to evaluate the effectiveness and cost-effectiveness of a telehealth intervention delivered by NHS Direct to support patients with LTCs. Two randomized controlled trials will be conducted in parallel, recruiting patients with two exemplar LTCs: depression or raised cardiovascular disease (CVD) risk. A total of 1,200 patients will be recruited from approximately 42 general practices near Bristol, Sheffield and Southampton, UK. Participants will be randomly allocated to either usual care (control group) or usual care plus the NHS Direct Healthlines Service (intervention group). The intervention is based on a conceptual model incorporating promotion of self-management, optimisation of treatment, coordination of care and engagement of patients and general practitioners. Participants will be provided with tailored help, combining telephone advice from health information advisors with support to use a range of online resources. Participants will access the service for 12 months. Outcomes will be collected at baseline, four, eight and 12 months for the depression trial and baseline, six and 12 months for the CVD risk trial. The primary outcome will be the proportion of patients responding to treatment, defined in the depression trial as a PHQ-9 score <10 and an absolute reduction in PHQ-9 ≥5 after 4 months, and in the CVD risk trial as maintenance or reduction of 10-year CVD risk after 12 months. The study will also assess whether the intervention is cost-effective from the perspective of the NHS and personal social services. An embedded qualitative interview study will explore healthcare professionals' and patients' views of the intervention.
Discussion: This study evaluates a complex telehealth intervention which combines evidence-based components and is delivered by an established healthcare organisation. The study will also analyse health economic information. In doing so, the study hopes to address some of the limitations of previous research by demonstrating the effectiveness and cost-effectiveness of a real world telehealth intervention.
Trial registration: Current Controlled Trials: Depression trial ISRCTN14172341 and cardiovascular disease risk trial ISRCTN27508731.
Figures
References
-
- Department of Health. Raising the Profile of Long Term Conditions Care: a compendium of information. London: Her Majesty’s Stationery Office (HMSO); January 2008. Available at http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.g... (accessed 10 January 2014)
-
- Department of Health. Long term conditions model. [ http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Healthcare/...]
-
- Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Hirani S, Cartwright M, Rixon L, Knapp M, Henderson C, Rogers A, Fitzpatrick R, Hendy J, Newman S. Whole System Demonstrator Evaluation Team. Effect of telehealth on use of secondary care and mortality: findings from the whole system demonstrator cluster randomised trial. BMJ. 2012;344:e3874. doi: 10.1136/bmj.e3874. - DOI - PMC - PubMed
-
- García-Lizana F, Sarría-Santamera A. New technologies for chronic disease management and control: a systematic review. J Telemed Telecare. 2007;13:62–68. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials