Comparative decellularization and recellularization of normal versus emphysematous human lungs
- PMID: 24461327
- PMCID: PMC4215725
- DOI: 10.1016/j.biomaterials.2013.12.103
Comparative decellularization and recellularization of normal versus emphysematous human lungs
Abstract
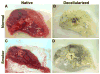
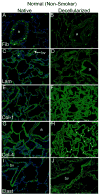
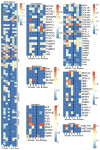
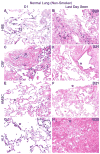
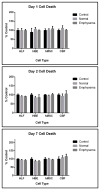
Acellular whole human lung scaffolds represent a unique opportunity for ex vivo tissue engineering. However, it remains unclear whether lungs from individuals with chronic lung diseases such as chronic obstructive pulmonary disease (COPD) can be appropriately decellularized and recellularized. To assess this, cadaveric human lungs from normal (non-smoking) patients and from patients with COPD (smoking history) were decellularized and found by histochemical and immunohistochemical staining, electron microscopy, and mass spectrometry to retain characteristic histological architecture and extracellular matrix components (ECM) reflecting either normal or COPD, particularly emphysematous, origin. Inoculation of human bronchial epithelial cells, endothelial progenitor cells, bone marrow-derived mesenchymal stem cells, and lung fibroblasts via airway or vascular routes into small, excised segments of the decellularized lungs demonstrated that normal lung scaffolds robustly supported initial engraftment and growth of each cell type for up to one month. In contrast, despite initial binding, all cell types inoculated into decellularized emphysematous lungs did not survive beyond one week. However, cell attachment and proliferation on solubilized ECM homogenates of decellularized normal and emphysematous lungs coated onto tissue culture plates was comparable and not impaired, suggesting that the 3-dimensional decellularized emphysematous scaffolds may lack the necessary ECM architecture to support sustained cell growth.
Keywords: Acellular matrix; Emphysema; Endothelial cell; Epithelial cell; Extracellular matrix (ECM); Human lung fibroblast.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures










References
-
- Orens JB, Garrity ER., Jr General overview of lung transplantation and review of organ allocation. Proc Am Thorac Soc. 2009;6:13–9. - PubMed
-
- Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 GM103449/GM/NIGMS NIH HHS/United States
- T32 HL076122/HL/NHLBI NIH HHS/United States
- RC4 HL106625/HL/NHLBI NIH HHS/United States
- RC4HL106625/HL/NHLBI NIH HHS/United States
- P20 RR-155557/RR/NCRR NIH HHS/United States
- P30 CA22435/CA/NCI NIH HHS/United States
- R21HL094611/HL/NHLBI NIH HHS/United States
- R21 HL108689/HL/NHLBI NIH HHS/United States
- P30 CA022435/CA/NCI NIH HHS/United States
- R21 HL094611/HL/NHLBI NIH HHS/United States
- 8P20GM103449/GM/NIGMS NIH HHS/United States
- R21 HL087274/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

