Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation
- PMID: 24461462
- PMCID: PMC4260089
- DOI: 10.1111/tpj.12445
Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation
Abstract
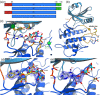
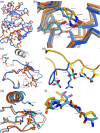
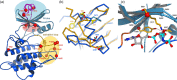
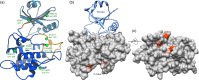
Brassinosteroids, which control plant growth and development, are sensed by the membrane receptor kinase BRASSINOSTEROID INSENSITIVE 1 (BRI1). Brassinosteroid binding to the BRI1 leucine-rich repeat (LRR) domain induces heteromerisation with a SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK)-family co-receptor. This process allows the cytoplasmic kinase domains of BRI1 and SERK to interact, trans-phosphorylate and activate each other. Here we report crystal structures of the BRI1 kinase domain in its activated form and in complex with nucleotides. BRI1 has structural features reminiscent of both serine/threonine and tyrosine kinases, providing insight into the evolution of dual-specificity kinases in plants. Phosphorylation of Thr1039, Ser1042 and Ser1044 causes formation of a catalytically competent activation loop. Mapping previously identified serine/threonine and tyrosine phosphorylation sites onto the structure, we analyse their contribution to brassinosteroid signaling. The location of known genetic missense alleles provide detailed insight into the BRI1 kinase mechanism, while our analyses are inconsistent with a previously reported guanylate cyclase activity. We identify a protein interaction surface on the C-terminal lobe of the kinase and demonstrate that the isolated BRI1, SERK2 and SERK3 cytoplasmic segments form homodimers in solution and have a weak tendency to heteromerise. We propose a model in which heterodimerisation of the BRI1 and SERK ectodomains brings their cytoplasmic kinase domains in a catalytically competent arrangement, an interaction that can be modulated by the BRI1 inhibitor protein BKI1.
Keywords: Arabidopsis thaliana; brassinosteroid receptor; growth control; hormone signaling; plant development; protein crystallography; protein phosphorylation; receptor kinase.
© 2014 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Figures

References
-
- Clark SE, Williams RW, Meyerowitz EM. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997;89:575–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

