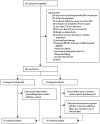
Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial
- PMID: 24461612
- PMCID: PMC4730945
- DOI: 10.1016/S2213-2600(13)70166-8
Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial
Erratum in
- Lancet Respir Med. 2013 Oct;1(8):592
Abstract
Background: Delirium is frequently diagnosed in critically ill patients and is associated with poor clinical outcomes. Haloperidol is the most commonly used drug for delirium despite little evidence of its effectiveness. The aim of this study was to establish whether early treatment with haloperidol would decrease the time that survivors of critical illness spent in delirium or coma.
Methods: We did this double-blind, placebo-controlled randomised trial in a general adult intensive care unit (ICU). Critically ill patients (≥18 years) needing mechanical ventilation within 72 h of admission were enrolled. Patients were randomised (by an independent nurse, in 1:1 ratio, with permuted block size of four and six, using a centralised, secure web-based randomisation service) to receive haloperidol 2.5 mg or 0.9% saline placebo intravenously every 8 h, irrespective of coma or delirium status. Study drug was discontinued on ICU discharge, once delirium-free and coma-free for 2 consecutive days, or after a maximum of 14 days of treatment, whichever came first. Delirium was assessed using the confusion assessment method for the ICU (CAM-ICU). The primary outcome was delirium-free and coma-free days, defined as the number of days in the first 14 days after randomisation during which the patient was alive without delirium and not in coma from any cause. Patients who died within the 14 day study period were recorded as having 0 days free of delirium and coma. ICU clinical and research staff and patients were masked to treatment throughout the study. Analyses were by intention to treat. This trial is registered with the International Standard Randomised Controlled Trial Registry, number ISRCTN83567338.
Findings: 142 patients were randomised, 141 were included in the final analysis (71 haloperidol, 70 placebo). Patients in the haloperidol group spent about the same number of days alive, without delirium, and without coma as did patients in the placebo group (median 5 days [IQR 0-10] vs 6 days [0-11] days; p=0.53). The most common adverse events were oversedation (11 patients in the haloperidol group vs six in the placebo group) and QTc prolongation (seven patients in the haloperidol group vs six in the placebo group). No patient had a serious adverse event related to the study drug.
Interpretation: These results do not support the hypothesis that haloperidol modifies duration of delirium in critically ill patients. Although haloperidol can be used safely in this population of patients, pending the results of trials in progress, the use of intravenous haloperidol should be reserved for short-term management of acute agitation.
Funding: National Institute for Health Research.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Can critical-care delirium be treated pharmacologically?Lancet Respir Med. 2013 Sep;1(7):498-9. doi: 10.1016/S2213-2600(13)70178-4. Epub 2013 Aug 21. Lancet Respir Med. 2013. PMID: 24461599 No abstract available.
-
Hope for haloperidol in delirium.Lancet Respir Med. 2013 Oct;1(8):e27. doi: 10.1016/S2213-2600(13)70210-8. Epub 2013 Oct 7. Lancet Respir Med. 2013. PMID: 24461669 No abstract available.
-
Prophylactic haloperidol: too early to lose hope.Lancet Respir Med. 2013 Oct;1(8):e27-e28. doi: 10.1016/S2213-2600(13)70193-0. Epub 2013 Oct 7. Lancet Respir Med. 2013. PMID: 24461670 No abstract available.
-
Prophylactic haloperidol: too early to lose hope - author's reply.Lancet Respir Med. 2013 Oct;1(8):e28. doi: 10.1016/S2213-2600(13)70194-2. Epub 2013 Oct 7. Lancet Respir Med. 2013. PMID: 24461671 No abstract available.
References
-
- Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. - PubMed
-
- Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73. - PubMed
-
- Peterson J, Pun B. Dittus R et al Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54(3):479–484. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical