Tropism and innate host responses of a novel avian influenza A H7N9 virus: an analysis of ex-vivo and in-vitro cultures of the human respiratory tract
- PMID: 24461614
- PMCID: PMC7164816
- DOI: 10.1016/S2213-2600(13)70138-3
Tropism and innate host responses of a novel avian influenza A H7N9 virus: an analysis of ex-vivo and in-vitro cultures of the human respiratory tract
Abstract
Background: Since March, 2013, an avian-origin influenza A H7N9 virus has caused severe pneumonia in China. The aim of this study was to investigate the pathogenesis of this new virus in human beings.
Methods: We obtained ex-vivo cultures of the human bronchus, lung, nasopharynx, and tonsil and in-vitro cultures of primary human alveolar epithelial cells and peripheral blood monocyte-derived macrophages. We compared virus tropism and induction of proinflammatory cytokine responses of two human influenza A H7N9 virus isolates, A/Shanghai/1/2013 and A/Shanghai/2/2013; a highly pathogenic avian influenza H5N1 virus; the highly pathogenic avian influenza H7N7 virus that infected human beings in the Netherlands in 2003; the 2009 pandemic influenza H1N1 virus, and a low pathogenic duck H7N9 virus that was genetically different to the human disease causing A H7N9 viruses.
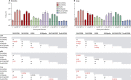
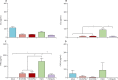
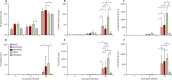
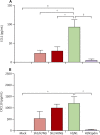
Findings: Both human H7N9 viruses replicated efficiently in human bronchus and lung ex-vivo cultures, whereas duck/H7N9 virus failed to replicate in either. Both human A H7N9 viruses infected both ciliated and non-ciliated human bronchial epithelial cells and replicated to higher titres than did H5N1 (p<0.0001 to 0.0046) and A/Shanghai/1/2013 replicated to higher titres than did H7N7 (p=0.0002-0.01). Both human A H7N9 viruses predominantly infected type II alveolar epithelial cells and alveolar macrophages in the human lung and replicated to higher titres than did H5N1 (p<0.0001 to 0.0078); A/Shanghai/1/2013 replicated to higher titres than did H1N1 (p=0.0052-0.05) and H7N7 (p=0.0031-0.0151). Human H7N9 viruses were less potent inducers of proinflammatory cytokines compared with H5N1 virus.
Interpretation: Collectively, the results suggest that the novel H7N9 viruses are better adapted to infect and replicate in the human conducting and lower airways than are other avian influenza viruses, including H5N1, and pose an important pandemic threat.
Funding: Area of Excellence Scheme of the University Grants Committee (AoE/M-12/96), Hong Kong Special Administrative Region.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Tropism of H7N9 influenza viruses in the human respiratory tract.Lancet Respir Med. 2013 Sep;1(7):501-2. doi: 10.1016/S2213-2600(13)70161-9. Epub 2013 Jul 25. Lancet Respir Med. 2013. PMID: 24461601 Free PMC article. No abstract available.
References
-
- Gao R, Cao B, Hu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. - PubMed
-
- WHO Human infection with avian influenza A(H7N9) virus—update. http://www.who.int/csr/don/2013_07_04/en/index.html (accessed July 7, 2013).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

