Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants
- PMID: 24461786
- PMCID: PMC3992195
- DOI: 10.1016/j.jpeds.2013.12.025
Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants
Erratum in
- J Pediatr. 2014 May;164(5):1244
Abstract
Objective: To examine the effect of early initiation of caffeine therapy on neonatal outcomes and characterize the use of caffeine therapy in very low birth weight (VLBW) infants.
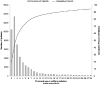
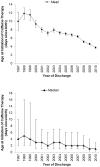
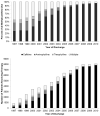
Study design: We analyzed a cohort of 62 056 VLBW infants discharged between 1997 and 2010 who received caffeine therapy. We compared outcomes in infants receiving early caffeine therapy (initial dose before 3 days of life) and those receiving late caffeine therapy (initial dose at or after 3 days of life) through propensity scoring using baseline and early clinical variables. The primary outcome was the association between the timing of caffeine initiation and the incidence of bronchopulmonary dysplasia (BPD) or death.
Results: We propensity score-matched 29 070 VLBW infants at a 1:1. Of infants receiving early caffeine therapy, 3681 (27.6%) died or developed BPD, compared with 4591 infants (34.0%) receiving late caffeine therapy (OR, 0.74; 99% CI, 0.69-0.80). Infants receiving early caffeine had a lower incidence of BPD (23.1% vs 30.7%; OR, 0.68; 95% CI, 0.63-0.73) and a higher incidence of death (4.5% vs 3.7%; OR, 1.23; 95% CI, 1.05-1.43). Infants receiving early caffeine therapy had less treatment of patent ductus arteriosus (OR, 0.60; 95% CI, 0.55-0.65) and a shorter duration of mechanical ventilation (mean difference, 6 days; P < .001).
Conclusion: Early caffeine initiation is associated with a decreased incidence of BPD. Randomized trials are needed to determine the efficacy and safety of early caffeine prophylaxis in VLBW infants.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Timing of caffeine therapy in very low birth weight infants.J Pediatr. 2014 May;164(5):957-8. doi: 10.1016/j.jpeds.2014.01.054. Epub 2014 Mar 12. J Pediatr. 2014. PMID: 24630349 No abstract available.
References
-
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–87. - PubMed
-
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–21. - PubMed
-
- Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–82. - PubMed
-
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. - PubMed
-
- Aranda JV, Turmen T, Davis J, Trippenbach T, Grondin D, Zinman R, et al. Effect of caffeine on control of breathing in infantile apnea. J Pediatr. 1983;103:975–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 HD060040/HD/NICHD NIH HHS/United States
- 1K23HL092225-01/HL/NHLBI NIH HHS/United States
- KL2TR000455/TR/NCATS NIH HHS/United States
- K23 HD068497/HD/NICHD NIH HHS/United States
- UL1TR001117/TR/NCATS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- HHSN267200700051C/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- L40 HD069892/HD/NICHD NIH HHS/United States
- KL2 TR000455/TR/NCATS NIH HHS/United States
- UL1TR000454/TR/NCATS NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- 1R18AE000028-01/AE/ASPE HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

