Opening paths to novel analgesics: the role of potassium channels in chronic pain
- PMID: 24461875
- PMCID: PMC3945816
- DOI: 10.1016/j.tins.2013.12.002
Opening paths to novel analgesics: the role of potassium channels in chronic pain
Abstract
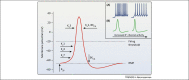
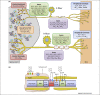
Chronic pain is associated with abnormal excitability of the somatosensory system and remains poorly treated in the clinic. Potassium (K⁺) channels are crucial determinants of neuronal activity throughout the nervous system. Opening of these channels facilitates a hyperpolarizing K⁺ efflux across the plasma membrane that counteracts inward ion conductance and therefore limits neuronal excitability. Accumulating research has highlighted a prominent involvement of K⁺ channels in nociceptive processing, particularly in determining peripheral hyperexcitability. We review salient findings from expression, pharmacological, and genetic studies that have untangled a hitherto undervalued contribution of K⁺ channels in maladaptive pain signaling. These emerging data provide a framework to explain enigmatic pain syndromes and to design novel pharmacological treatments for these debilitating states.
Keywords: dorsal root ganglia; pain; pharmacotherapy; potassium channel.
Crown Copyright © 2014. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- van Hecke O. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013;111:13–18. - PubMed
-
- Labianca R. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin. Drug Investig. 2012;32(Suppl. 1):53–63. - PubMed
-
- D’Mello R., Dickenson A.H. Spinal cord mechanisms of pain. Br. J. Anaesth. 2008;101:8–16. - PubMed
-
- Kajander K.C. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci. Lett. 1992;138:225–228. - PubMed
-
- Kajander K.C., Bennett G.J. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in Abeta and Adelta primary afferent neurons. J. Neurophysiol. 1992;68:734–744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

