Histopathologic and immunohistochemical sequelae of bariatric embolization in a porcine model
- PMID: 24462005
- PMCID: PMC4040229
- DOI: 10.1016/j.jvir.2013.09.016
Histopathologic and immunohistochemical sequelae of bariatric embolization in a porcine model
Abstract
Purpose: To evaluate the histopathologic sequelae of bariatric embolization on the gastric mucosa and to correlate with immunohistochemical evaluation of the gastric fundus, antrum, and duodenum.
Materials and methods: This study was performed on 12 swine stomach and duodenum specimens after necropsy. Of the 12 swine, 6 had previously undergone bariatric embolization of the gastric fundus, and the 6 control swine had undergone a sham procedure with saline. Gross pathologic, histopathologic, and immunohistochemical examinations of the stomach and duodenum were performed. Specifically, mucosal integrity, fibrosis, ghrelin-expressing cells, and gastrin-expressing cells were assessed.
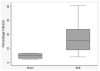
Results: Gross and histopathologic evaluation of treatment animals showed healing or healed mucosal ulcers in 50% of animals, with gastritis in 100% of treatment animals and in five of six control animals. The ghrelin-immunoreactive mean cell density was significantly lower in the gastric fundus in the treated animals compared with control animals (15.3 vs 22.0, P < .01) but similar in the gastric antrum (9.3 vs 14.3, P = .08) and duodenum (8.5 vs 8.6, P = .89). The gastrin-expressing cell density was significantly lower in the antrum of treated animals compared with control animals (82.2 vs 126.4, P = .03). A trend toward increased fibrosis was suggested in the gastric fundus of treated animals compared with controls (P = .07).
Conclusions: Bariatric embolization resulted in a significant reduction in ghrelin-expressing cells in the gastric fundus without evidence of upregulation of ghrelin-expressing cells in the duodenum. Healing ulcerations in half of treated animals underscores the need for additional refinement of this procedure.
© 2014 Published by SIR on behalf of The Society of Interventional Radiology.
Conflict of interest statement
A.A. is a consultant and shareholder with Surefire Medical. None of the other authors have identified a conflict of interest.
Figures





References
-
- Druce MR, Small CJ, Bloom SR. Minireview: Gut peptides regulating satiety. Endocrinology. 2004;145:2660–2665. - PubMed
-
- Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. - PubMed
-
- Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–456. - PubMed
-
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. - PubMed
-
- Hayashida T, Murakami K, Mogi K, et al. Ghrelin in domestic animals: distribution in stomach and its possible role. Domestic Anim Endocrinol. 2001;21:17–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

