Reactive gliosis and the multicellular response to CNS damage and disease
- PMID: 24462092
- PMCID: PMC3984950
- DOI: 10.1016/j.neuron.2013.12.034
Reactive gliosis and the multicellular response to CNS damage and disease
Abstract
The CNS is prone to heterogeneous insults of diverse etiologies that elicit multifaceted responses. Acute and focal injuries trigger wound repair with tissue replacement. Diffuse and chronic diseases provoke gradually escalating tissue changes. The responses to CNS insults involve complex interactions among cells of numerous lineages and functions, including CNS intrinsic neural cells, CNS intrinsic nonneural cells, and CNS extrinsic cells that enter from the circulation. The contributions of diverse nonneuronal cell types to outcome after acute injury, or to the progression of chronic disease, are of increasing interest as the push toward understanding and ameliorating CNS afflictions accelerates. In some cases, considerable information is available, in others, comparatively little, as examined and reviewed here.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

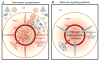

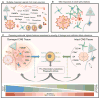
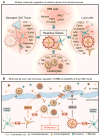

References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

