Dividing cells regulate their lipid composition and localization
- PMID: 24462247
- PMCID: PMC3909459
- DOI: 10.1016/j.cell.2013.12.015
Dividing cells regulate their lipid composition and localization
Abstract
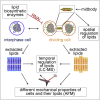
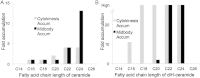
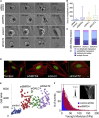
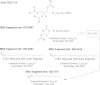
Although massive membrane rearrangements occur during cell division, little is known about specific roles that lipids might play in this process. We report that the lipidome changes with the cell cycle. LC-MS-based lipid profiling shows that 11 lipids with specific chemical structures accumulate in dividing cells. Using AFM, we demonstrate differences in the mechanical properties of live dividing cells and their isolated lipids relative to nondividing cells. In parallel, systematic RNAi knockdown of lipid biosynthetic enzymes identified enzymes required for division, which highly correlated with lipids accumulated in dividing cells. We show that cells specifically regulate the localization of lipids to midbodies, membrane-based structures where cleavage occurs. We conclude that cells actively regulate and modulate their lipid composition and localization during division, with both signaling and structural roles likely. This work has broader implications for the active and sustained participation of lipids in basic biology.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Comment in
-
The changing lipidome during cell division.Cell. 2014 Jan 30;156(3):394-5. doi: 10.1016/j.cell.2014.01.018. Cell. 2014. PMID: 24485447
References
Supplemental References
-
- Cases S., Stone S.J., Zhou P., Yen E., Tow B., Lardizabal K.D., Voelker T., Farese R.V., Jr. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 2001;276:38870–38876. - PubMed
-
- Ivanova P.T., Milne S.B., Forrester J.S., Brown H.A. LIPID arrays: new tools in the understanding of membrane dynamics and lipid signaling. Mol. Interv. 2004;4:86–96. - PubMed
-
- Kihara T., Yoshida T., Haghparast S.M.A., Miyake J. Elasticity Mapping Analysis of Apical Cell Periphery Actin Structures of Normal Fibroblasts and Cervical Cancer Cells. JASMI. 2013;3:124–129.
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM082834/GM/NIGMS NIH HHS/United States
- BB/J00992X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 GM039565/GM/NIGMS NIH HHS/United States
- R01 GM39565/GM/NIGMS NIH HHS/United States
- J00992X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

