Activation of cutaneous immune responses in complex regional pain syndrome
- PMID: 24462502
- PMCID: PMC4011956
- DOI: 10.1016/j.jpain.2014.01.490
Activation of cutaneous immune responses in complex regional pain syndrome
Abstract
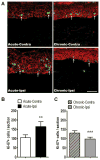
The pathogenesis of complex regional pain syndrome (CRPS) is unresolved, but tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) are elevated in experimental skin blister fluid from CRPS-affected limbs, as is tryptase, a marker for mast cells. In the rat fracture model of CRPS, exaggerated sensory and sympathetic neural signaling stimulate keratinocyte and mast cell proliferation, causing the local production of high levels of inflammatory cytokines leading to pain behavior. The current investigation used CRPS patient skin biopsies to determine whether keratinocyte and mast cell proliferation occur in CRPS skin and to identify the cellular source of the up-regulated TNF-α, IL-6, and tryptase observed in CRPS experimental skin blister fluid. Skin biopsies were collected from the affected skin and the contralateral mirror site in 55 CRPS patients and the biopsy sections were immunostained for keratinocyte, cell proliferation, mast cell markers, TNF-α, and IL-6. In early CRPS, keratinocytes were activated in the affected skin, resulting in proliferation, epidermal thickening, and up-regulated TNF-α and IL-6 expression. In chronic CRPS, there was reduced keratinocyte proliferation, leading to epidermal thinning in the affected skin. Acute CRPS patients also had increased mast cell accumulation in the affected skin, but there was no increase in mast cell numbers in chronic CRPS.
Perspective: The results of this study support the hypotheses that CRPS involves activation of the innate immune system, with keratinocyte and mast cell activation and proliferation, inflammatory mediator release, and pain.
Keywords: Complex regional pain syndrome; immunology; keratinocytes; mast cells; pain.
Published by Elsevier Inc.
Conflict of interest statement
Figures





References
-
- Bernateck M, Karst M, Gratz KF, Meyer GJ, Fischer MJ, Knapp WH, Koppert W, Brunkhorst T. The first scintigraphic detection of tumor necrosis factor-alpha in patients with complex regional pain syndrome type 1. Anesthesia and analgesia. 2010;110:211–215. - PubMed
-
- Bernateck M, Rolke R, Birklein F, Treede RD, Fink M, Karst M. Successful intravenous regional block with low-dose tumor necrosis factor-alpha antibody infliximab for treatment of complex regional pain syndrome 1. Anesthesia and analgesia. 2007;105:1148–1151. - PubMed
-
- Birklein F, Riedl B, Sieweke N, Weber M, Neundorfer B. Neurological findings in complex regional pain syndromes--analysis of 145 cases. Acta Neurol Scand. 2000;101:262–269. - PubMed
-
- Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113:713–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

