BDNF secretion by human pulmonary artery endothelial cells in response to hypoxia
- PMID: 24462831
- PMCID: PMC3977651
- DOI: 10.1016/j.yjmcc.2014.01.006
BDNF secretion by human pulmonary artery endothelial cells in response to hypoxia
Abstract
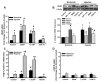
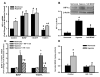
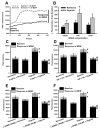
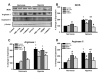
Within human pulmonary artery, neurotrophin growth factors [NTs; e.g. brain-derived neurotrophic factor (BDNF)] and their high-affinity receptors (tropomyosin-related kinase; Trk) and low-affinity receptors p75 neurotrophin receptor (p75NTR) have been reported, but their functional role is incompletely understood. We tested the hypothesis that BDNF is produced by human pulmonary artery endothelial cells (PAECs). In the context of hypoxia as a risk factor for pulmonary hypertension, we examined the effect of hypoxia on BDNF secretion and consequent autocrine effects on pulmonary endothelium. Initial ELISA analysis of circulating BDNF in 30 healthy human volunteers showed that 72 h exposure to high altitude (~11,000 ft, alveolar PO2 = 100 mmHg) results in higher BDNF compared to samples taken at sea level. Separately, in human PAECs exposed for 24h to normoxia vs. hypoxia (1-3% O2), ELISA of extracellular media showed increased BDNF levels. Furthermore, quantitative PCR of PAECs showed 3-fold enhancement of BDNF gene transcription with hypoxia. In PAECs, BDNF induced NO production (measured using an NO-sensitive fluorescent dye DAF2-DA) that was significantly higher under hypoxic conditions, an effect also noted with the TrkB agonist 7,8-DHF. Importantly, hypoxia-induced NO was blunted by neutralization of secreted BDNF using the chimeric TrkB-Fc. Both hypoxia and BDNF increased iNOS (but not eNOS) mRNA expression. In accordance, BDNF enhancement of NO in hypoxia was not blunted by 50 nM L-NAME (eNOS inhibition) but substantially lower with 100 μM L-NAME (eNOS and iNOS inhibition). Hypoxia and BDNF also induced expression of hypoxia inducible factor 1 alpha (HIF-1α), a subunit of the transcription factor HIF-1, and pharmacological inhibition of HIF-1 diminished hypoxia effects on BDNF expression and secretion, and NO production. These results indicate that human PAECs express and secrete BDNF in response to hypoxia via a HIF-1-regulated pathway.
Keywords: Hypoxia inducible factor 1; Neurotrophin; Nitric oxide; Tropomyosin related kinase; eNOS; iNOS.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
