Dkk2/Frzb in the dermal papillae regulates feather regeneration
- PMID: 24463139
- PMCID: PMC4384700
- DOI: 10.1016/j.ydbio.2014.01.010
Dkk2/Frzb in the dermal papillae regulates feather regeneration
Abstract
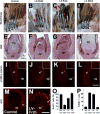
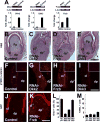
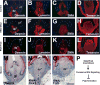
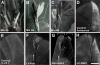
Avian feathers have robust growth and regeneration capability. To evaluate the contribution of signaling molecules and pathways in these processes, we profiled gene expression in the feather follicle using an absolute quantification approach. We identified hundreds of genes that mark specific components of the feather follicle: the dermal papillae (DP) which controls feather regeneration and axis formation, the pulp mesenchyme (Pp) which is derived from DP cells and nourishes the feather follicle, and the ramogenic zone epithelium (Erz) where a feather starts to branch. The feather DP is enriched in BMP/TGF-β signaling molecules and inhibitors for Wnt signaling including Dkk2/Frzb. Wnt ligands are mainly expressed in the feather epithelium and pulp. We find that while Wnt signaling is required for the maintenance of DP marker gene expression and feather regeneration, excessive Wnt signaling delays regeneration and reduces pulp formation. Manipulating Dkk2/Frzb expression by lentiviral-mediated overexpression, shRNA-knockdown, or by antibody neutralization resulted in dual feather axes formation. Our results suggest that the Wnt signaling in the proximal feather follicle is fine-tuned to accommodate feather regeneration and axis formation.
Keywords: Axis formation; Dermal papillae; Dkk2/Dkk3/Frzb; Feather follicle; Regeneration.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Long non-coding RNAs regulate Wnt signaling during feather regeneration.Development. 2018 Oct 29;145(21):dev162388. doi: 10.1242/dev.162388. Development. 2018. PMID: 30327322
-
Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia.Proc Natl Acad Sci U S A. 2006 Jan 24;103(4):951-5. doi: 10.1073/pnas.0506894103. Epub 2006 Jan 17. Proc Natl Acad Sci U S A. 2006. PMID: 16418297 Free PMC article.
-
Dermal FOXO3 activity in response to Wnt/β-catenin signaling is required for feather follicle development of goose embryos (Anser cygnoides).Poult Sci. 2024 Mar;103(3):103424. doi: 10.1016/j.psj.2024.103424. Epub 2024 Jan 11. Poult Sci. 2024. PMID: 38330682 Free PMC article.
-
Feather regeneration as a model for organogenesis.Dev Growth Differ. 2013 Jan;55(1):139-48. doi: 10.1111/dgd.12024. Dev Growth Differ. 2013. PMID: 23294361 Free PMC article. Review.
-
Molecular Regulatory Mechanisms in Chicken Feather Follicle Morphogenesis.Genes (Basel). 2023 Aug 18;14(8):1646. doi: 10.3390/genes14081646. Genes (Basel). 2023. PMID: 37628697 Free PMC article. Review.
Cited by
-
The Modulatable Stem Cell Niche: Tissue Interactions during Hair and Feather Follicle Regeneration.J Mol Biol. 2016 Apr 10;428(7):1423-40. doi: 10.1016/j.jmb.2015.07.009. Epub 2015 Jul 18. J Mol Biol. 2016. PMID: 26196442 Free PMC article. Review.
-
Transition from natal downs to juvenile feathers: conserved regulatory switches in Neoaves.Res Sq [Preprint]. 2023 Oct 3:rs.3.rs-3382427. doi: 10.21203/rs.3.rs-3382427/v1. Res Sq. 2023. Update in: Nat Commun. 2024 May 16;15(1):4174. doi: 10.1038/s41467-024-48303-3. PMID: 37886492 Free PMC article. Updated. Preprint.
-
Contraction of basal filopodia controls periodic feather branching via Notch and FGF signaling.Nat Commun. 2018 Apr 9;9(1):1345. doi: 10.1038/s41467-018-03801-z. Nat Commun. 2018. PMID: 29632339 Free PMC article.
-
Modulation of tooth regeneration through opposing responses to Wnt and BMP signals in teleosts.Development. 2023 Dec 1;150(23):dev202168. doi: 10.1242/dev.202168. Epub 2023 Dec 7. Development. 2023. PMID: 38059590 Free PMC article.
-
Integration Analysis of Transcriptome Sequencing and Whole-Genome Resequencing Reveal Wool Quality-Associated Key Genes in Zhexi Angora Rabbits.Vet Sci. 2024 Dec 13;11(12):651. doi: 10.3390/vetsci11120651. Vet Sci. 2024. PMID: 39728991 Free PMC article.
References
-
- Andl T, Reddy ST, Gaddapara T, Millar SE. Wnt signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. - PubMed
-
- Clavel C, Grisanti L, Zemla R, Rezza A, Barros R, Sennett R, Mazloom AR, Chung CY, Cai X, Cai CL, Pevny L, Nicolis S, Ma’ayan A, Rendl M. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell. 2012;23:981–994. - PMC - PubMed
-
- Das RM, Van Hateren NJ, Howell GR, Farrell ER, Bangs FK, Porteous VC, Manning EM, McGrew MJ, Ohyama K, Sacco MA, Halley PA, Sang HM, Storey KG, Placzek M, Tickle C, Nair VK, Wilson SA. A robust system for RNA interference in the chicken using a modified microRNA operon. Dev Biol. 2006;294:554–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

