Microgravity control of autophagy modulates osteoclastogenesis
- PMID: 24463210
- PMCID: PMC4384509
- DOI: 10.1016/j.bone.2014.01.004
Microgravity control of autophagy modulates osteoclastogenesis
Abstract
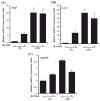
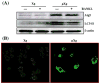
Evidence indicates that astronauts experience significant bone loss during space mission. Recently, we used the NASA developed rotary cell culture system (RCCS) to simulate microgravity (μXg) conditions and demonstrated increased osteoclastogenesis in mouse bone marrow cultures. Autophagy is a cellular recycling process of nutrients. Therefore, we hypothesize that μXg control of autophagy modulates osteoclastogenesis. Real-time PCR analysis of total RNA isolated from mouse bone marrow derived non-adherent cells subjected to modeled μXg showed a significant increase in autophagic marker Atg5, LC3 and Atg16L mRNA expression compared to ground based control (Xg) cultures. Western blot analysis of total cell lysates identified an 8.0-fold and 7.0-fold increase in the Atg5 and LC3-II expression, respectively. Confocal microscopy demonstrated an increased autophagosome formation in μXg subjected RAW 264.7 preosteoclast cells. RT(2) profiler PCR array screening for autophagy related genes identified that μXg upregulates intracellular signaling molecules associated with autophagy, autophagosome components and inflammatory cytokines/growth factors which coregulate autophagy in RAW 264.7 preosteoclast cells. Autophagy inhibitor, 3-methyladenine (3-MA) treatment of mouse bone marrow derived non-adherent mononuclear cells showed a significant decrease in μXg induced Atg5 and LC3 mRNA expression in the presence or absence of RANK ligand (RANKL) stimulation. Furthermore, RANKL treatment significantly increased (8-fold) p-CREB transcription factor levels under μXg as compared to Xg cultures and 3-MA inhibited RANKL increased p-CREB expression in these cells. Also, 3-MA suppresses μXg elevated osteoclast differentiation in mouse bone marrow cultures. Thus, our results suggest that μXg induced autophagy plays an important role in enhanced osteoclast differentiation and could be a potential therapeutic target to prevent bone loss in astronauts during space flight missions.
Keywords: Autophagy; Microgravity; NASA; Osteoclast; Rotary cell culture system (RCCS).
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Carmeliet G, Bouillon R. The effect of microgravity on morphology and gene expression of osteoblasts in vitro. FASEB J. 1999;13(Suppl):S129–34. - PubMed
-
- Sibonga JD. Spaceflight-induced bone loss: is there an osteoporosis risk? Curr Osteoporos Rep. 2013;11:92–8. - PubMed
-
- Garber MA, McDowell DL, Hutton WC. Bone loss during simulated weightlessness: a biomechanical and mineralization study in the rat model. Aviat Space Environ Med. 2000;71:586–92. - PubMed
-
- Lang TF, Leblanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res. 2006;21:1224–30. - PubMed
-
- Cavanagh PR, Licata AA, Rice AJ. Exercise and pharmacological countermeasures for bone loss during long-duration space flight. Gravit Space Biol Bull. 2005;18:39–58. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

