Sorting out the trash: the spatial nature of eukaryotic protein quality control
- PMID: 24463332
- PMCID: PMC4204729
- DOI: 10.1016/j.ceb.2013.12.006
Sorting out the trash: the spatial nature of eukaryotic protein quality control
Abstract
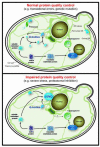
Failure to maintain protein homeostasis is associated with aggregation and cell death, and underies a growing list of pathologies including neurodegenerative diseases, aging, and cancer. Misfolded proteins can be toxic and interfere with normal cellular functions, particularly during proteotoxic stress. Accordingly, molecular chaperones, the ubiquitin-proteasome system (UPS) and autophagy together promote refolding or clearance of misfolded proteins. Here we discuss emerging evidence that the pathways of protein quality control (PQC) are intimately linked to cell architecture, and sequester proteins into spatially and functionally distinct PQC compartments. This sequestration serves a number of functions, including enhancing the efficiency of quality control; clearing the cellular milieu of potentially toxic species and facilitating asymmetric inheritance of damaged proteins to promote rejuvenation of daughter cells.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. - PubMed
-
- Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2010;40:238–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

