Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation
- PMID: 24463369
- PMCID: PMC3981906
- DOI: 10.1161/CIRCULATIONAHA.113.004742
Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation
Abstract
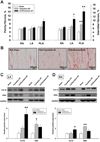
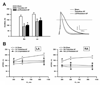
Background: Little is known about the mechanisms underlying the transition from paroxysmal to persistent atrial fibrillation (AF). In an ovine model of long-standing persistent AF we tested the hypothesis that the rate of electric and structural remodeling, assessed by dominant frequency (DF) changes, determines the time at which AF becomes persistent.
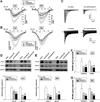
Methods and results: Self-sustained AF was induced by atrial tachypacing. Seven sheep were euthanized 11.5±2.3 days after the transition to persistent AF and without reversal to sinus rhythm; 7 sheep were euthanized after 341.3±16.7 days of long-standing persistent AF. Seven sham-operated animals were in sinus rhythm for 1 year. DF was monitored continuously in each group. Real-time polymerase chain reaction, Western blotting, patch clamping, and histological analyses were used to determine the changes in functional ion channel expression and structural remodeling. Atrial dilatation, mitral valve regurgitation, myocyte hypertrophy, and atrial fibrosis occurred progressively and became statistically significant after the transition to persistent AF, with no evidence for left ventricular dysfunction. DF increased progressively during the paroxysmal-to-persistent AF transition and stabilized when AF became persistent. Importantly, the rate of DF increase correlated strongly with the time to persistent AF. Significant action potential duration abbreviation, secondary to functional ion channel protein expression changes (CaV1.2, NaV1.5, and KV4.2 decrease; Kir2.3 increase), was already present at the transition and persisted for 1 year of follow up.
Conclusions: In the sheep model of long-standing persistent AF, the rate of DF increase predicts the time at which AF stabilizes and becomes persistent, reflecting changes in action potential duration and densities of sodium, L-type calcium, and inward rectifier currents.
Keywords: atrial fibrillation; electrophysiological; fibrosis; ion channels; refractory period.
Conflict of interest statement
Figures





References
-
- de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. - PubMed
-
- Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. - PubMed
-
- Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial l-type ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. - PubMed
-
- Caballero R, de la Fuente MG, Gomez R, Barana A, Amoros I, Dolz-Gaiton P, Osuna L, Almendral J, Atienza F, Fernandez-Aviles F, Pita A, Rodriguez-Roda J, Pinto A, Tamargo J, Delpon E. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J Am Coll Cardiol. 2010;55:2346–2354. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

