NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation
- PMID: 24463449
- PMCID: PMC3904618
- DOI: 10.1172/JCI70812
NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation
Abstract
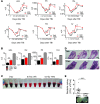
A nuclear disaster may result in exposure to potentially lethal doses of ionizing radiation (IR). Hematopoietic acute radiation syndrome (H-ARS) is characterized by severe myelosuppression, which increases the risk of infection, bleeding, and mortality. Here, we determined that activation of nuclear factor erythroid-2-related factor 2 (NRF2) signaling enhances hematopoietic stem progenitor cell (HSPC) function and mitigates IR-induced myelosuppression and mortality. Augmenting NRF2 signaling in mice, either by genetic deletion of the NRF2 inhibitor Keap1 or by pharmacological NRF2 activation with 2-trifluoromethyl-2'-methoxychalone (TMC), enhanced hematopoietic reconstitution following bone marrow transplantation (BMT). Strikingly, even 24 hours after lethal IR exposure, oral administration of TMC mitigated myelosuppression and mortality in mice. Furthermore, TMC administration to irradiated transgenic Notch reporter mice revealed activation of Notch signaling in HSPCs and enhanced HSPC expansion by increasing Jagged1 expression in BM stromal cells. Administration of a Notch inhibitor ablated the effects of TMC on hematopoietic reconstitution. Taken together, we identified a mechanism by which NRF2-mediated Notch signaling improves HSPC function and myelosuppression following IR exposure. Our data indicate that targeting this pathway may provide a countermeasure against the damaging effects of IR exposure.
Figures


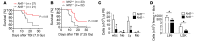



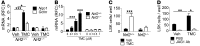
Comment in
-
NRF2 mitigates radiation-induced hematopoietic death.J Clin Invest. 2014 Mar;124(3):960-1. doi: 10.1172/JCI74143. Epub 2014 Feb 24. J Clin Invest. 2014. PMID: 24569364 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL105569/HL/NHLBI NIH HHS/United States
- P50HL107169/HL/NHLBI NIH HHS/United States
- R33AI080541/AI/NIAID NIH HHS/United States
- P01 ES018176/ES/NIEHS NIH HHS/United States
- P50 CA058184/CA/NCI NIH HHS/United States
- U01HL105569/HL/NHLBI NIH HHS/United States
- U01 AI107361/AI/NIAID NIH HHS/United States
- R01 CA140492/CA/NCI NIH HHS/United States
- ES03819/ES/NIEHS NIH HHS/United States
- P50 HL107169/HL/NHLBI NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- P50ES015903/ES/NIEHS NIH HHS/United States
- U01AI107361/AI/NIAID NIH HHS/United States
- R33 AI080541/AI/NIAID NIH HHS/United States
- P01ES018176/ES/NIEHS NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- P50 ES015903/ES/NIEHS NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- P30 ES003819/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

