Evaluation of teriparatide treatment in adults with osteogenesis imperfecta
- PMID: 24463451
- PMCID: PMC3904621
- DOI: 10.1172/JCI71101
Evaluation of teriparatide treatment in adults with osteogenesis imperfecta
Abstract
Background: Adults with osteogenesis imperfecta (OI) have a high risk of fracture. Currently, few treatment options are available, and bone anabolic therapies have not been tested in clinical trials for OI treatment.
Methods: 79 adults with OI were randomized to receive 20 μg recombinant human parathyroid hormone (teriparatide) or placebo for 18 months in a double-blind, placebo-controlled trial. The primary endpoint was the percent change in areal bone mineral density (aBMD) of the lumbar spine (LS), as determined by dual-energy X-ray absorptiometry. Secondary endpoints included percent change in bone remodeling markers and vertebral volumetric BMD (vBMD) by quantitative computed tomography, estimated vertebral strength by finite element analysis, and self-reported fractures.
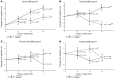
Results: Compared with the placebo group, the teriparatide group showed increased LS aBMD (6.1% ± 1.0% vs. 2.8% ± 1.0% change from baseline; P < 0.05) and total hip aBMD (2.6% ± 1.0% vs. -2.4% ± 1.0% change; P < 0.001). Vertebral vBMD and strength improved with teriparatide therapy (18% ± 6% and 15% ± 3% change, respectively), but declined with placebo (-5.0% ± 6% and -2.0% ± 3% change; P < 0.05 for both comparisons). Serum procollagen type 1 N-terminal propeptide (P1NP) and urine collagen N-telopeptide (NTx) levels increased with teriparatide therapy (135% ± 14% and 64% ± 10% change, respectively). Teriparatide-induced elevation of P1NP levels was less pronounced in severe forms of OI (type III/IV) compared with the milder form (type I). Type I OI patients exhibited robust BMD increases with teriparatide; however, there was no observed benefit for those with type III/IV OI. There was no difference in self-reported fractures between the 2 groups.
Conclusions: Adults with OI, particularly those with less severe disease (type I), displayed a teriparatide-induced anabolic response, as well as increased hip and spine aBMD, vertebral vBMD, and estimated vertebral strength. Trial registration. Clinicaltrials.gov NCT00131469. Funding. The Osteoporosis Imperfecta Foundation, Eli Lilly and Co., the National Center for Advancing Translational Science (NCATS) at the NIH (grant no. UL1RR024140), and the Baylor College of Medicine General Clinical Research Center (grant no. RR00188).
Figures


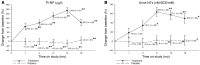
Comment in
- J Clin Invest. 124:476.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

