Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection
- PMID: 24463452
- PMCID: PMC3904624
- DOI: 10.1172/JCI71858
Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection
Abstract
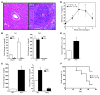
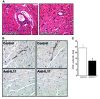
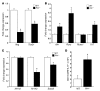
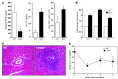
Th cells are the major effector cells in transplant rejection and can be divided into Th1, Th2, Th17, and Treg subsets. Th differentiation is controlled by transcription factor expression, which is driven by positive and negative cytokine and chemokine stimuli at the time of T cell activation. Here we discovered that chemokine platelet factor 4 (PF4) is a negative regulator of Th17 differentiation. PF4-deficient and platelet-deficient mice had exaggerated immune responses to cardiac transplantation, including increased numbers of infiltrating Th17 cells and increased plasma IL-17. Although PF4 has been described as a platelet-specific molecule, we found that activated T cells also express PF4. Furthermore, bone marrow transplantation experiments revealed that T cell-derived PF4 contributes to a restriction in Th17 differentiation. Taken together, the results of this study demonstrate that PF4 is a key regulator of Th cell development that is necessary to limit Th17 differentiation. These data likely will impact our understanding of platelet-dependent regulation of T cell development, which is important in many diseases, in addition to transplantation.
Figures

Comment in
-
An unexpected role for platelets in blocking Th17 differentiation.J Clin Invest. 2014 Feb;124(2):480-2. doi: 10.1172/JCI74231. Epub 2014 Jan 27. J Clin Invest. 2014. PMID: 24463445 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

