Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients
- PMID: 24463454
- PMCID: PMC3904627
- DOI: 10.1172/JCI72227
Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients
Erratum in
- J Clin Invest. 2014 Apr 1;124(4):1868
Abstract
Background: Sodium-glucose cotransporter 2 (SGLT2) inhibitors lower glycemia by enhancing urinary glucose excretion. The physiologic response to pharmacologically induced acute or chronic glycosuria has not been investigated in human diabetes.
Methods: We evaluated 66 patients with type 2 diabetes (62 ± 7 years, BMI = 31.6 ± 4.6 kg/m(2), HbA1c = 55 ± 8 mmol/mol, mean ± SD) at baseline, after a single dose, and following 4-week treatment with empagliflozin (25 mg). At each time point, patients received a mixed meal coupled with dual-tracer glucose administration and indirect calorimetry.
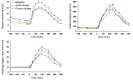
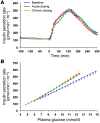
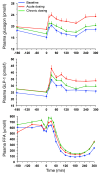
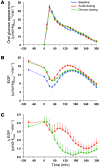
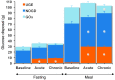
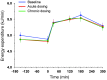
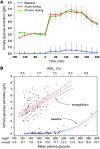
Results: Both single-dose and chronic empagliflozin treatment caused glycosuria during fasting (median, 7.8 [interquartile range {IQR}, 4.4] g/3 hours and 9.2 [IQR, 5.2] g/3 hours) and after meal ingestion (median, 29.0 [IQR, 12.5] g/5 hours and 28.2 [IQR, 15.4] g/5 hours). After 3 hours of fasting, endogenous glucose production (EGP) was increased 25%, while glycemia was 0.9 ± 0.7 mmol/l lower (P < 0.0001 vs. baseline). After meal ingestion, glucose and insulin AUC decreased, whereas the glucagon response increased (all P < 0.001). While oral glucose appearance was unchanged, EGP was increased (median, 40 [IQR, 14] g and 37 [IQR, 11] g vs. 34 [IQR, 11] g, both P < 0.01). Tissue glucose disposal was reduced (median, 75 [IQR, 16] g and 70 [IQR, 21] g vs. 93 [IQR, 18] g, P < 0.0001), due to a decrease in both glucose oxidation and nonoxidative glucose disposal, with a concomitant rise in lipid oxidation after chronic administration (all P < 0.01). β Cell glucose sensitivity increased (median, 55 [IQR, 35] pmol • min(-1) • m(-2) • mM(-1) and 55 [IQR, 39] pmol • min(-1) • m(-2) • mM(-1) vs. 44 [IQR, 32] pmol • min(-1) • m(-2) • mM(-1), P < 0.0001), and insulin sensitivity was improved. Resting energy expenditure rates and those after meal ingestion were unchanged.
Conclusions: In patients with type 2 diabetes, empagliflozin-induced glycosuria improved β cell function and insulin sensitivity, despite the fall in insulin secretion and tissue glucose disposal and the rise in EGP after one dose, thereby lowering fasting and postprandial glycemia. Chronic dosing shifted substrate utilization from carbohydrate to lipid. Trial registration. ClinicalTrials.Gov NCT01248364 (EudraCT no. 2010-018708-99). Funding. This study was funded by Boehringer Ingelheim.
Figures
Comment in
-
Paradoxical insights into whole body metabolic adaptations following SGLT2 inhibition.J Clin Invest. 2014 Feb;124(2):485-7. doi: 10.1172/JCI74297. Epub 2014 Jan 27. J Clin Invest. 2014. PMID: 24463446 Free PMC article.
References
-
- Yki-Järvinen H. Glucose toxicity. Endocr Rev. 1992;13(3):415–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

