A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes
- PMID: 24463512
- PMCID: PMC4282169
- DOI: 10.1038/nature12907
A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes
Abstract
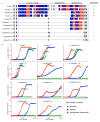
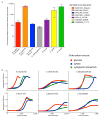
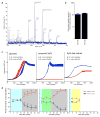
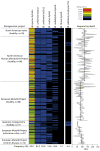
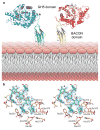
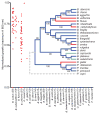
A well-balanced human diet includes a significant intake of non-starch polysaccharides, collectively termed 'dietary fibre', from the cell walls of diverse fruits and vegetables. Owing to the paucity of alimentary enzymes encoded by the human genome, our ability to derive energy from dietary fibre depends on the saccharification and fermentation of complex carbohydrates by the massive microbial community residing in our distal gut. The xyloglucans (XyGs) are a ubiquitous family of highly branched plant cell wall polysaccharides whose mechanism(s) of degradation in the human gut and consequent importance in nutrition have been unclear. Here we demonstrate that a single, complex gene locus in Bacteroides ovatus confers XyG catabolism in this common colonic symbiont. Through targeted gene disruption, biochemical analysis of all predicted glycoside hydrolases and carbohydrate-binding proteins, and three-dimensional structural determination of the vanguard endo-xyloglucanase, we reveal the molecular mechanisms through which XyGs are hydrolysed to component monosaccharides for further metabolism. We also observe that orthologous XyG utilization loci (XyGULs) serve as genetic markers of XyG catabolism in Bacteroidetes, that XyGULs are restricted to a limited number of phylogenetically diverse strains, and that XyGULs are ubiquitous in surveyed human metagenomes. Our findings reveal that the metabolism of even highly abundant components of dietary fibre may be mediated by niche species, which has immediate fundamental and practical implications for gut symbiont population ecology in the context of human diet, nutrition and health.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




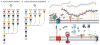
Comment in
-
How do gut microbes break down dietary fiber?Trends Biochem Sci. 2014 Apr;39(4):156-8. doi: 10.1016/j.tibs.2014.02.005. Epub 2014 Mar 5. Trends Biochem Sci. 2014. PMID: 24613530
References
-
- McDougall GJ, Morrison IM, Stewart D, Hillman JR. Plant cell walls as dietary fibre: Range, structure, processing and function. J Sci Food Agric. 1996;70:133–150.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

