Recent developments in copper and zinc homeostasis in bacterial pathogens
- PMID: 24463765
- PMCID: PMC4008645
- DOI: 10.1016/j.cbpa.2013.12.021
Recent developments in copper and zinc homeostasis in bacterial pathogens
Abstract
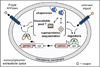
Copper and zinc homeostasis systems in pathogenic bacteria are required to resist host efforts to manipulate the availability and toxicity of these metal ions. Central to this microbial adaptive response is the involvement of metal-trafficking and metal-sensing proteins that ultimately exercise control of metal speciation in the cell. Cu-specific and Zn-specific metalloregulatory proteins regulate the transcription of metal-responsive genes while metallochaperones and related proteins ensure that these metals are appropriately buffered by the intracellular milieu and delivered to correct intracellular targets. In this review, we summarize recent findings on how bacterial pathogens mount a metal-specific response to derail host efforts to win the 'fight over metals.'
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



References
-
- Kozlowski H, Janicka-Klos A, Brasun J, Gaggelli E, Valensin D, Valensin G. Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation) Coord Chem Rev. 2009;253:2665–2685.
-
- Weinberg ED. Nutritional immunity: host's attempt to withhold iron from microbial invaders. J Am Med Assoc. 1975;231:39–41. - PubMed
-
-
Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. A comprehensive review of how the host restricts the availability of some essential metals and induces toxicity with others in order to kill invading bacterial pathogens.
-
-
- Botella H, Stadthagen G, Lugo-Villarino G, de Chastellier C, Neyrolles O. Metallobiology of host-pathogen interactions: an intoxicating new insight. Trends Microbiol. 2012;20:106–112. - PubMed
-
- Plüddemann A, Mukhopadhyay S, Gordon S. Innate immunity to intracellular pathogens: macrophage receptors and responses to microbial entry. Immunol Rev. 2011;240:11–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

