HDAC1 activates FoxO and is both sufficient and required for skeletal muscle atrophy
- PMID: 24463822
- PMCID: PMC3970557
- DOI: 10.1242/jcs.136390
HDAC1 activates FoxO and is both sufficient and required for skeletal muscle atrophy
Abstract
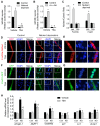
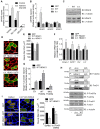
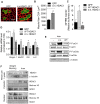
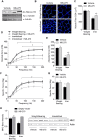
The Forkhead box O (FoxO) transcription factors are activated, and necessary for the muscle atrophy, in several pathophysiological conditions, including muscle disuse and cancer cachexia. However, the mechanisms that lead to FoxO activation are not well defined. Recent data from our laboratory and others indicate that the activity of FoxO is repressed under basal conditions via reversible lysine acetylation, which becomes compromised during catabolic conditions. Therefore, we aimed to determine how histone deacetylase (HDAC) proteins contribute to activation of FoxO and induction of the muscle atrophy program. Through the use of various pharmacological inhibitors to block HDAC activity, we demonstrate that class I HDACs are key regulators of FoxO and the muscle-atrophy program during both nutrient deprivation and skeletal muscle disuse. Furthermore, we demonstrate, through the use of wild-type and dominant-negative HDAC1 expression plasmids, that HDAC1 is sufficient to activate FoxO and induce muscle fiber atrophy in vivo and is necessary for the atrophy of muscle fibers that is associated with muscle disuse. The ability of HDAC1 to cause muscle atrophy required its deacetylase activity and was linked to the induction of several atrophy genes by HDAC1, including atrogin-1, which required deacetylation of FoxO3a. Moreover, pharmacological inhibition of class I HDACs during muscle disuse, using MS-275, significantly attenuated both disuse muscle fiber atrophy and contractile dysfunction. Together, these data solidify the importance of class I HDACs in the muscle atrophy program and indicate that class I HDAC inhibitors are feasible countermeasures to impede muscle atrophy and weakness.
Keywords: Acetylation; Deacetylation; FoxO; Histone deacetylase; Muscle disuse; Nutrient deprivation.
Figures

References
-
- Caiozzo V. J., Haddad F., Baker M. J., Herrick R. E., Prietto N., Baldwin K. M. (1996). Microgravity-induced transformations of myosin isoforms and contractile properties of skeletal muscle. J. Appl. Physiol. 81, 123–132 - PubMed
-
- Caiozzo V. J., Baker M. J., Baldwin K. M. (1998). Novel transitions in MHC isoforms: separate and combined effects of thyroid hormone and mechanical unloading. J. Appl. Physiol. 85, 2237–2248 - PubMed
-
- Campione M., Ausoni S., Guezennec C. Y., Schiaffino S. (1993). Myosin and troponin changes in rat soleus muscle after hindlimb suspension. J. Appl. Physiol. 74, 1156–1160 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

