A functional genomics screen identifies PCAF and ADA3 as regulators of human granzyme B-mediated apoptosis and Bid cleavage
- PMID: 24464226
- PMCID: PMC3978306
- DOI: 10.1038/cdd.2013.203
A functional genomics screen identifies PCAF and ADA3 as regulators of human granzyme B-mediated apoptosis and Bid cleavage
Abstract
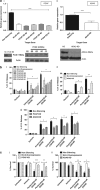
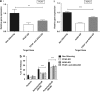
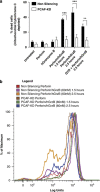
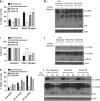
The human lymphocyte toxins granzyme B (hGrzB) and perforin cooperatively induce apoptosis of virus-infected or transformed cells: perforin pores enable entry of the serine protease hGrzB into the cytosol, where it processes Bid to selectively activate the intrinsic apoptosis pathway. Truncated Bid (tBid) induces Bax/Bak-dependent mitochondrial outer membrane permeability and the release of cytochrome c and Smac/Diablo. To identify cellular proteins that regulate perforin/hGrzB-mediated Bid cleavage and subsequent apoptosis, we performed a gene-knockdown (KD) screen using a lentiviral pool of short hairpin RNAs embedded within a miR30 backbone (shRNAmiR). We transduced HeLa cells with a lentiviral pool expressing shRNAmiRs that target 1213 genes known to be involved in cell death signaling and selected cells with acquired resistance to perforin/hGrzB-mediated apoptosis. Twenty-two shRNAmiRs were identified in the positive-selection screen including two, PCAF and ADA3, whose gene products are known to reside in the same epigenetic regulatory complexes. Small interfering (si)RNA-mediated gene-KD of PCAF or ADA3 also conferred resistance to perforin/hGrzB-mediated apoptosis providing independent validation of the screen results. Mechanistically, PCAF and ADA3 exerted their pro-apoptotic effect upstream of mitochondrial membrane permeabilization, as indicated by reduced cytochrome c release in PCAF-KD cells exposed to perforin/hGrzB. While overall levels of Bid were unaltered, perforin/hGrzB-mediated cleavage of Bid was reduced in PCAF-KD or ADA3-KD cells. We discovered that PCAF-KD or ADA3-KD resulted in reduced expression of PACS2, a protein implicated in Bid trafficking to mitochondria and importantly, targeted PACS2-KD phenocopied the effect of PCAF-KD or ADA3-KD. We conclude that PCAF and ADA3 regulate Bid processing via PACS2, to modulate the mitochondrial cell death pathway in response to hGrzB.
Figures




References
-
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. - PubMed
-
- Alimonti JB, Shi L, Baijal PK, Greenberg AH. Granzyme B induces BID-mediated cytochrome c release and mitochondrial permeability transition. J Biol Chem. 2001;276:6974–6982. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

