Aging genomes: a necessary evil in the logic of life
- PMID: 24464418
- PMCID: PMC5985526
- DOI: 10.1002/bies.201300127
Aging genomes: a necessary evil in the logic of life
Abstract
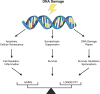
Genomes are inherently unstable because of the need for DNA sequence variation as a substrate for evolution through natural selection. However, most multicellular organisms have postmitotic tissues, with limited opportunity for selective removal of cells harboring persistent damage and deleterious mutations, which can therefore contribute to functional decline, disease, and death. Key in this process is the role of genome maintenance, the network of protein products that repair DNA damage and signal DNA damage response pathways. Genome maintenance is beneficial early in life by swiftly eliminating DNA damage or damaged cells, facilitating rapid cell proliferation. However, at later ages accumulation of unrepaired damage and mutations, as well as ongoing cell depletion, promotes cancer, atrophy, and other deleterious effects associated with aging. As such, genome maintenance and its phenotypic sequelae provide yet another example of antagonistic pleiotropy in aging and longevity.
Keywords: DNA damage; DNA epimutations; DNA mutations; DNA repair; aging; evolution.
© 2014 WILEY Periodicals, Inc.
Conflict of interest statement
The author has declared no conflict of interest.
Figures



Similar articles
-
DNA Damage, DNA Repair, Aging, and Neurodegeneration.Cold Spring Harb Perspect Med. 2015 Sep 18;5(10):a025130. doi: 10.1101/cshperspect.a025130. Cold Spring Harb Perspect Med. 2015. PMID: 26385091 Free PMC article. Review.
-
Genome instability and aging.Annu Rev Physiol. 2013;75:645-68. doi: 10.1146/annurev-physiol-030212-183715. Annu Rev Physiol. 2013. PMID: 23398157 Review.
-
Protecting the Aging Genome.Trends Cell Biol. 2020 Feb;30(2):117-132. doi: 10.1016/j.tcb.2019.12.001. Epub 2020 Jan 6. Trends Cell Biol. 2020. PMID: 31917080 Review.
-
From DNA damage to mutations: All roads lead to aging.Ageing Res Rev. 2021 Jul;68:101316. doi: 10.1016/j.arr.2021.101316. Epub 2021 Mar 9. Ageing Res Rev. 2021. PMID: 33711511 Free PMC article. Review.
-
Genome instability, cancer and aging.Biochim Biophys Acta. 2009 Oct;1790(10):963-9. doi: 10.1016/j.bbagen.2009.03.020. Epub 2009 Mar 31. Biochim Biophys Acta. 2009. PMID: 19344750 Free PMC article. Review.
Cited by
-
DNA Damage, DNA Repair, Aging, and Neurodegeneration.Cold Spring Harb Perspect Med. 2015 Sep 18;5(10):a025130. doi: 10.1101/cshperspect.a025130. Cold Spring Harb Perspect Med. 2015. PMID: 26385091 Free PMC article. Review.
-
Enforced DNA repair enzymes rescue neurons from apoptosis induced by target deprivation and axotomy in mouse models of neurodegeneration.Mech Ageing Dev. 2017 Jan;161(Pt A):149-162. doi: 10.1016/j.mad.2016.06.011. Epub 2016 Jun 27. Mech Ageing Dev. 2017. PMID: 27364693 Free PMC article.
-
Nutritional Preconditioning in Cancer Treatment in Relation to DNA Damage and Aging.Annu Rev Cancer Biol. 2021 Mar;5:161-179. doi: 10.1146/annurev-cancerbio-060820-090737. Epub 2020 Dec 2. Annu Rev Cancer Biol. 2021. PMID: 35474917 Free PMC article.
-
Regenerative Medicine: Shedding Light on the Link between Aging and Cancer.Cell Transplant. 2017 Sep;26(9):1530-1537. doi: 10.1177/0963689717721224. Cell Transplant. 2017. PMID: 29113461 Free PMC article. Review.
-
Circuit mechanisms encoding odors and driving aging-associated behavioral declines in Caenorhabditis elegans.Elife. 2015 Sep 22;4:e10181. doi: 10.7554/eLife.10181. Elife. 2015. PMID: 26394000 Free PMC article.
References
-
- Bernstein H, Hopf FA, Michod RE. The molecular basis of the evolution of sex. Adv Genet. 1987;24:323–70. - PubMed
-
- de Duve C. The onset of selection. Nature. 2005;433:581–2. - PubMed
-
- Hart RW, Sacher GA, Hoskins TL. DNA repair in a short- and a long-lived rodent species. J Gerontol. 1979;34:808–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

