Nonclassical transpeptidases of Mycobacterium tuberculosis alter cell size, morphology, the cytosolic matrix, protein localization, virulence, and resistance to β-lactams
- PMID: 24464457
- PMCID: PMC3993333
- DOI: 10.1128/JB.01396-13
Nonclassical transpeptidases of Mycobacterium tuberculosis alter cell size, morphology, the cytosolic matrix, protein localization, virulence, and resistance to β-lactams
Abstract
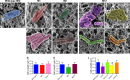
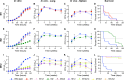
Virtually all bacteria possess a peptidoglycan layer that is essential for their growth and survival. The β-lactams, the most widely used class of antibiotics in human history, inhibit D,D-transpeptidases, which catalyze the final step in peptidoglycan biosynthesis. The existence of a second class of transpeptidases, the L,D-transpeptidases, was recently reported. Mycobacterium tuberculosis, an infectious pathogen that causes tuberculosis (TB), is known to possess as many as five proteins with L,D-transpeptidase activity. Here, for the first time, we demonstrate that loss of L,D-transpeptidases 1 and 2 of M. tuberculosis (LdtMt1 and LdtMt2) alters cell surface morphology, shape, size, organization of the intracellular matrix, sorting of some low-molecular-weight proteins that are targeted to the membrane or secreted, cellular physiology, growth, virulence, and resistance of M. tuberculosis to amoxicillin-clavulanate and vancomycin.
Figures



Similar articles
-
Non-classical transpeptidases yield insight into new antibacterials.Nat Chem Biol. 2017 Jan;13(1):54-61. doi: 10.1038/nchembio.2237. Epub 2016 Nov 7. Nat Chem Biol. 2017. PMID: 27820797 Free PMC article.
-
Structures of free and inhibited forms of the L,D-transpeptidase LdtMt1 from Mycobacterium tuberculosis.Acta Crystallogr D Biol Crystallogr. 2013 Sep;69(Pt 9):1697-706. doi: 10.1107/S0907444913013085. Epub 2013 Aug 15. Acta Crystallogr D Biol Crystallogr. 2013. PMID: 23999293
-
In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by L,D-transpeptidases and inactivation of these enzymes by carbapenems.Antimicrob Agents Chemother. 2013 Dec;57(12):5940-5. doi: 10.1128/AAC.01663-13. Epub 2013 Sep 16. Antimicrob Agents Chemother. 2013. PMID: 24041897 Free PMC article.
-
Fluorescent probes for investigating peptidoglycan biosynthesis in mycobacteria.Curr Opin Chem Biol. 2020 Aug;57:50-57. doi: 10.1016/j.cbpa.2020.04.006. Epub 2020 Jun 9. Curr Opin Chem Biol. 2020. PMID: 32531742 Review.
-
Structure and Function of L,D- and D,D-Transpeptidase Family Enzymes from Mycobacterium tuberculosis.Curr Med Chem. 2020;27(19):3250-3267. doi: 10.2174/0929867326666181203150231. Curr Med Chem. 2020. PMID: 30501595 Review.
Cited by
-
First Penicillin-Binding Protein Occupancy Patterns for 15 β-Lactams and β-Lactamase Inhibitors in Mycobacterium abscessus.Antimicrob Agents Chemother. 2020 Dec 16;65(1):e01956-20. doi: 10.1128/AAC.01956-20. Print 2020 Dec 16. Antimicrob Agents Chemother. 2020. PMID: 33106266 Free PMC article.
-
Coxiella burnetii RpoS Regulates Genes Involved in Morphological Differentiation and Intracellular Growth.J Bacteriol. 2019 Mar 26;201(8):e00009-19. doi: 10.1128/JB.00009-19. Print 2019 Apr 15. J Bacteriol. 2019. PMID: 30745369 Free PMC article.
-
Altered Mycobacterium tuberculosis Cell Wall Metabolism and Physiology Associated With RpoB Mutation H526D.Front Microbiol. 2018 Mar 19;9:494. doi: 10.3389/fmicb.2018.00494. eCollection 2018. Front Microbiol. 2018. PMID: 29616007 Free PMC article.
-
Accumulation of Peptidoglycan O-Acetylation Leads to Altered Cell Wall Biochemistry and Negatively Impacts Pathogenesis Factors of Campylobacter jejuni.J Biol Chem. 2016 Oct 21;291(43):22686-22702. doi: 10.1074/jbc.M116.746404. Epub 2016 Jul 29. J Biol Chem. 2016. PMID: 27474744 Free PMC article.
-
Activity-Based Protein Profiling Reveals That Cephalosporins Selectively Active on Non-replicating Mycobacterium tuberculosis Bind Multiple Protein Families and Spare Peptidoglycan Transpeptidases.Front Microbiol. 2020 Jun 23;11:1248. doi: 10.3389/fmicb.2020.01248. eCollection 2020. Front Microbiol. 2020. PMID: 32655524 Free PMC article.
References
-
- Crick DC, Brennan PJ. 2008. Biosynthesis of the arabinogalactan-peptidoglycan complex, p 25–40 In Daffe M, Reyrat J. (ed), The mycobacterial cell envelope. ASM Press, Washington, DC
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

