Novel role of aminopeptidase-A in angiotensin-(1-7) metabolism post myocardial infarction
- PMID: 24464749
- PMCID: PMC3962633
- DOI: 10.1152/ajpheart.00911.2013
Novel role of aminopeptidase-A in angiotensin-(1-7) metabolism post myocardial infarction
Abstract
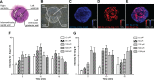
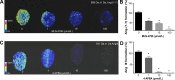
Aminopeptidase-A (APA) is a less well-studied enzyme of the renin-angiotensin system. We propose that it is involved in cardiac angiotensin (ANG) metabolism and its pathologies. ANG-(1-7) can ameliorate remodeling after myocardial injury. The aims of this study are to (1) develop mass spectrometric (MS) approaches for the assessment of ANG processing by APA within the myocardium; and (2) investigate the role of APA in cardiac ANG-(1-7) metabolism after myocardial infarction (MI) using sensitive MS techniques. MI was induced in C57Bl/6 male mice by ligating the left anterior descending (LAD) artery. Frozen mouse heart sections (in situ assay) or myocardial homogenates (in vitro assay) were incubated with the endogenous APA substrate, ANG II. Results showed concentration- and time-dependent cardiac formation of ANG III from ANG II, which was inhibited by the specific APA inhibitor, 4-amino-4-phosphonobutyric acid. Myocardial APA activity was significantly increased 24 h after LAD ligation (0.82 ± 0.02 vs. 0.32 ± 0.02 ρmol·min(-1)·μg(-1), MI vs. sham, P < 0.01). Both MS enzyme assays identified the presence of a new peptide, ANG-(2-7), m/z 784, which accumulated in the MI (146.45 ± 6.4 vs. 72.96 ± 7.0%, MI vs. sham, P < 0.05). Use of recombinant APA enzyme revealed that APA is responsible for ANG-(2-7) formation from ANG-(1-7). APA exhibited similar substrate affinity for ANG-(1-7) compared with ANG II {Km (ANG II) = 14.67 ± 1.6 vs. Km [ANG-(1-7)] = 6.07 ± 1.12 μmol/l, P < 0.05}. Results demonstrate a novel role of APA in ANG-(1-7) metabolism and suggest that the upregulation of APA, which occurs after MI, may deprive the heart of cardioprotective ANG-(1-7). Thus APA may serve as a potentially novel therapeutic target for management of tissue remodeling after MI.
Keywords: MALDI-imaging; aminopeptidase-A; angiotensin peptides; myocardial infarction; renin-angiotensin system.
Figures






References
-
- Alghamri MS, Weir NM, Anstadt MP, Elased KM, Gurley SB, Morris M. Enhanced angiotensin II-induced cardiac and aortic remodeling in ACE2 knockout mice. J Cardiovasc Pharmacol Ther 18: 138–151, 2013 - PubMed
-
- Bodineau L, Frugiere A, Marc Y, Claperon C, Llorens-Cortes C. Aminopeptidase A inhibitors as centrally acting antihypertensive agents. Heart Fail Rev 13: 311–319, 2008 - PubMed
-
- Bodineau L, Frugiere A, Marc Y, Inguimbert N, Fassot C, Balavoine F, Roques B, Llorens-Cortes C. Orally active aminopeptidase A inhibitors reduce blood pressure: a new strategy for treating hypertension. Hypertension 51: 1318–1325, 2008 - PubMed
-
- Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res 62: 415–425, 2004 - PubMed
-
- Clark MA, Nguyen C, Tran H. Angiotensin III induces c-Jun N-terminal kinase leading to proliferation of rat astrocytes. Neurochem Res 37: 1475–1481, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

