Equilibrium Study of Pd(dba)2 and P(OPh)3 in the Pd-Catalyzed Allylation of Aniline by Allyl Alcohol
- PMID: 24465076
- PMCID: PMC3893935
- DOI: 10.1021/om4009873
Equilibrium Study of Pd(dba)2 and P(OPh)3 in the Pd-Catalyzed Allylation of Aniline by Allyl Alcohol
Abstract
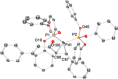
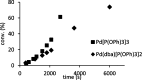
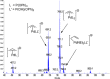
Reaction of Pd(dba)2 and P(OPh)3 shows a unique equilibrium where the Pd[P(OPh)3]3 complex is favored over both Pd(dba)[P(OPh)3]2 and Pd[P(OPh)3]4 complexes at room temperature. At a lower temperature, Pd[P(OPh)3]4 becomes the most abundant complex in solution. X-ray studies of Pd[P(OPh)3]3 and Pd(dba)[P(OPh)3]2 complexes show that both complexes have a trigonal geometry with a Pd-P distance of 2.25 Å due to the π-acidity of the phosphite ligand. In solution, pure Pd(dba)[P(OPh)3]2 complex equilibrates to the favored Pd[P(OPh)3]3 complex, which is the most stable complex of those studied, and also forms the most active catalytic species. This catalyst precursor dissociates one ligand to give the reactive Pd[P(OPh)3]2, which performs an oxidative addition of nonmanipulated allyl alcohol to generate the π-allyl-Pd[P(OPh)3]2 intermediate according to ESI-MS studies.
Figures
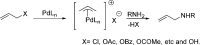




References
-
- Byström S. E.; Aslanian R.; Bäckvall J.-E. Tetrahedron Lett. 1985, 26, 1749.
- Dubovyk I.; Watson I. D. G.; Yudin A. K. J. Am. Chem. Soc. 2007, 129, 14172. - PubMed
- Nagano T.; Kobayashi S. J. Am. Chem. Soc. 2009, 131, 4200. - PubMed
- Nishikata T.; Lipshutz B. H. Chem. Commun. 2009, 6472. - PMC - PubMed
- Moreno-Mañas M.; Morral L.; Pleixats R. J. Org. Chem. 1998, 63, 6160. - PubMed
-
- Sheldon R. A. Pure Appl. Chem. 2000, 72, 1233.
- Trost B. M. Science 1991, 254, 1471. - PubMed
-
- Tamaru Y. Eur. J. Org. Chem. 2005, 2647.
- Muzart J. Tetrahedron 2005, 61, 4179.
- Muzart J. Eur. J. Org. Chem. 2007, 3077.
-
- Yang S.-C; Tsai Y.-C. Organometallics 2001, 20, 763.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
