Nonindustry-sponsored preclinical studies on statins yield greater efficacy estimates than industry-sponsored studies: a meta-analysis
- PMID: 24465178
- PMCID: PMC3897361
- DOI: 10.1371/journal.pbio.1001770
Nonindustry-sponsored preclinical studies on statins yield greater efficacy estimates than industry-sponsored studies: a meta-analysis
Abstract
Industry-sponsored clinical drug studies are associated with publication of outcomes that favor the sponsor, even when controlling for potential bias in the methods used. However, the influence of sponsorship bias has not been examined in preclinical animal studies. We performed a meta-analysis of preclinical statin studies to determine whether industry sponsorship is associated with either increased effect sizes of efficacy outcomes and/or risks of bias in a cohort of published preclinical statin studies. We searched Medline (January 1966-April 2012) and identified 63 studies evaluating the effects of statins on atherosclerosis outcomes in animals. Two coders independently extracted study design criteria aimed at reducing bias, results for all relevant outcomes, sponsorship source, and investigator financial ties. The I(2) statistic was used to examine heterogeneity. We calculated the standardized mean difference (SMD) for each outcome and pooled data across studies to estimate the pooled average SMD using random effects models. In a priori subgroup analyses, we assessed statin efficacy by outcome measured, sponsorship source, presence or absence of financial conflict information, use of an optimal time window for outcome assessment, accounting for all animals, inclusion criteria, blinding, and randomization. The effect of statins was significantly larger for studies sponsored by nonindustry sources (-1.99; 95% CI -2.68, -1.31) versus studies sponsored by industry (-0.73; 95% CI -1.00, -0.47) (p value<0.001). Statin efficacy did not differ by disclosure of financial conflict information, use of an optimal time window for outcome assessment, accounting for all animals, inclusion criteria, blinding, and randomization. Possible reasons for the differences between nonindustry- and industry-sponsored studies, such as selective reporting of outcomes, require further study.
Conflict of interest statement
The authors have declared that no competing interests exist.
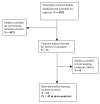
Figures

References
-
- Lo B, Field M (2009) COI in medical research, education, and practice. Washington, DC: The National Academies Press Institute of Medicine. - PubMed
-
- Higgins JP, Green S (2008) Cochrane handbook for systematic reviews of interventions. West Sussex, England: John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex.
-
- Bebarta V, Luyten D, Heard K (2003) Emergency medicine animal research: does use of randomization and blinding affect the results? Acad Emerg Med 10: 684–687. - PubMed
-
- Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, et al. (2008) Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke 39: 929–934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

