Bayesian reconstruction of disease outbreaks by combining epidemiologic and genomic data
- PMID: 24465202
- PMCID: PMC3900386
- DOI: 10.1371/journal.pcbi.1003457
Bayesian reconstruction of disease outbreaks by combining epidemiologic and genomic data
Abstract
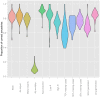
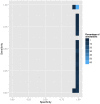
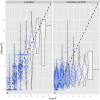
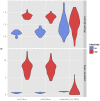
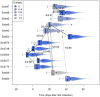
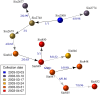
Recent years have seen progress in the development of statistically rigorous frameworks to infer outbreak transmission trees ("who infected whom") from epidemiological and genetic data. Making use of pathogen genome sequences in such analyses remains a challenge, however, with a variety of heuristic approaches having been explored to date. We introduce a statistical method exploiting both pathogen sequences and collection dates to unravel the dynamics of densely sampled outbreaks. Our approach identifies likely transmission events and infers dates of infections, unobserved cases and separate introductions of the disease. It also proves useful for inferring numbers of secondary infections and identifying heterogeneous infectivity and super-spreaders. After testing our approach using simulations, we illustrate the method with the analysis of the beginning of the 2003 Singaporean outbreak of Severe Acute Respiratory Syndrome (SARS), providing new insights into the early stage of this epidemic. Our approach is the first tool for disease outbreak reconstruction from genetic data widely available as free software, the R package outbreaker. It is applicable to various densely sampled epidemics, and improves previous approaches by detecting unobserved and imported cases, as well as allowing multiple introductions of the pathogen. Because of its generality, we believe this method will become a tool of choice for the analysis of densely sampled disease outbreaks, and will form a rigorous framework for subsequent methodological developments.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

