HIV-1 accessory proteins adapt cellular adaptors to facilitate immune evasion
- PMID: 24465204
- PMCID: PMC3900642
- DOI: 10.1371/journal.ppat.1003851
HIV-1 accessory proteins adapt cellular adaptors to facilitate immune evasion
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Collins K, Chen B, Kalams S, Walker B, Baltimore D (1998) HIV-1 Nef protein protects infected primary human cells from killing by cytotoxic T lymphocytes. Nature 391: 397–401. - PubMed
-
- Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM (1996) Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 2: 338–342. - PubMed
-
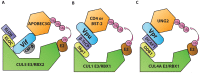
- Le Gall S, Erdtmann L, Benichou S, Berlloz-Torrent C, Liu L, et al. (1998) Nef interacts with mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8: 483–495. - PubMed
-
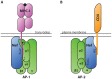
- Wonderlich ER, Williams M, Collins KL (2008) The tyrosine binding pocket in the adaptor protein 1 (AP-1) mu1 subunit is necessary for Nef to recruit AP-1 to the major histocompatibility complex class I cytoplasmic tail. J Biol Chem 283: 3011–3022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

