Intranasal vaccination promotes detrimental Th17-mediated immunity against influenza infection
- PMID: 24465206
- PMCID: PMC3900655
- DOI: 10.1371/journal.ppat.1003875
Intranasal vaccination promotes detrimental Th17-mediated immunity against influenza infection
Abstract
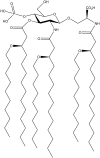
Influenza disease is a global health issue that causes significant morbidity and mortality through seasonal epidemics. Currently, inactivated influenza virus vaccines given intramuscularly or live attenuated influenza virus vaccines administered intranasally are the only approved options for vaccination against influenza virus in humans. We evaluated the efficacy of a synthetic toll-like receptor 4 agonist CRX-601 as an adjuvant for enhancing vaccine-induced protection against influenza infection. Intranasal administration of CRX-601 adjuvant combined with detergent split-influenza antigen (A/Uruguay/716/2007 (H3N2)) generated strong local and systemic immunity against co-administered influenza antigens while exhibiting high efficacy against two heterotypic influenza challenges. Intranasal vaccination with CRX-601 adjuvanted vaccines promoted antigen-specific IgG and IgA antibody responses and the generation of polyfunctional antigen-specific Th17 cells (CD4(+)IL-17A(+)TNFα(+)). Following challenge with influenza virus, vaccinated mice transiently exhibited increased weight loss and morbidity during early stages of disease but eventually controlled infection. This disease exacerbation following influenza infection in vaccinated mice was dependent on both the route of vaccination and the addition of the adjuvant. Neutralization of IL-17A confirmed a detrimental role for this cytokine during influenza infection. The expansion of vaccine-primed Th17 cells during influenza infection was also accompanied by an augmented lung neutrophilic response, which was partially responsible for mediating the increased morbidity. This discovery is of significance in the field of vaccinology, as it highlights the importance of both route of vaccination and adjuvant selection in vaccine development.
Conflict of interest statement
All authors were employed by the GlaxoSmithKline group of companies GSK Vaccines during completion of the experimental studies contained herein. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures
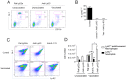
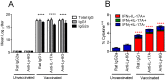
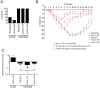

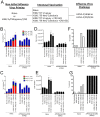
Similar articles
-
Effects of different adjuvants in the context of intramuscular and intranasal routes on humoral and cellular immune responses induced by detergent-split A/H3N2 influenza vaccines in mice.Clin Vaccine Immunol. 2012 Feb;19(2):209-18. doi: 10.1128/CVI.05441-11. Epub 2011 Dec 21. Clin Vaccine Immunol. 2012. PMID: 22190392 Free PMC article.
-
Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration.Vaccine. 2012 Jul 6;30(32):4884-91. doi: 10.1016/j.vaccine.2012.04.032. Epub 2012 Apr 23. Vaccine. 2012. PMID: 22537989
-
A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung.Vaccine. 2014 May 30;32(26):3216-22. doi: 10.1016/j.vaccine.2014.04.011. Epub 2014 Apr 13. Vaccine. 2014. PMID: 24731807
-
Studies on the usefulness of intranasal inactivated influenza vaccines.Vaccine. 2010 Aug 31;28(38):6393-7. doi: 10.1016/j.vaccine.2010.05.019. Epub 2010 May 20. Vaccine. 2010. PMID: 20493820 Review.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
Cited by
-
Adjuvantation of Pulmonary-Administered Influenza Vaccine with GPI-0100 Primarily Stimulates Antibody Production and Memory B Cell Proliferation.Vaccines (Basel). 2017 Jul 27;5(3):19. doi: 10.3390/vaccines5030019. Vaccines (Basel). 2017. PMID: 28749414 Free PMC article.
-
The neutralizing breadth of antibodies targeting diverse conserved epitopes between SARS-CoV and SARS-CoV-2.Proc Natl Acad Sci U S A. 2022 Aug 23;119(34):e2204256119. doi: 10.1073/pnas.2204256119. Epub 2022 Aug 16. Proc Natl Acad Sci U S A. 2022. PMID: 35972965 Free PMC article.
-
Abdominal and Pelvic Organ Failure Induced by Intraperitoneal Influenza A Virus Infection in Mice.Front Microbiol. 2020 Jul 17;11:1713. doi: 10.3389/fmicb.2020.01713. eCollection 2020. Front Microbiol. 2020. PMID: 32765481 Free PMC article.
-
Topical CpG Oligodeoxynucleotide Adjuvant Enhances the Adaptive Immune Response against Influenza A Infections.Front Immunol. 2016 Jul 29;7:284. doi: 10.3389/fimmu.2016.00284. eCollection 2016. Front Immunol. 2016. PMID: 27524984 Free PMC article.
-
Advancing Adjuvants for Mycobacterium tuberculosis Therapeutics.Front Immunol. 2021 Oct 25;12:740117. doi: 10.3389/fimmu.2021.740117. eCollection 2021. Front Immunol. 2021. PMID: 34759923 Free PMC article. Review.
References
-
- Cox RJ, Brokstad KA, Ogra P (2004) Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 59: 1–15. - PubMed
-
- de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD (2000) Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol 61: 94–99. - PubMed
-
- Pavot V, Rochereau N, Genin C, Verrier B, Paul S (2012) New insights in mucosal vaccine development. Vaccine 30: 142–154. - PubMed
-
- Osterholm MT, Kelley NS, Sommer A, Belongia EA (2012) Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12: 36–44. - PubMed
-
- Baldridge JR, Yorgensen Y, Ward JR, Ulrich JT (2000) Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine 18: 2416–2425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

