An Attenuated CMV Vaccine with a Deletion in Tegument Protein GP83 (pp65 Homolog) Protects against Placental Infection and Improves Pregnancy Outcome in a Guinea Pig Challenge Model
- PMID: 24465269
- PMCID: PMC3900008
- DOI: 10.2217/fvl.13.107
An Attenuated CMV Vaccine with a Deletion in Tegument Protein GP83 (pp65 Homolog) Protects against Placental Infection and Improves Pregnancy Outcome in a Guinea Pig Challenge Model
Abstract
Aims: Congenital human cytomegalovirus (HCMV) infection can lead to long-term neurodevelopmental sequelae, including mental retardation and sensorineural hearing loss. Preconception vaccine strategies relevant to prevention of HCMV-mediated injury to the newborn can be studied in the guinea pig cytomegalovirus (GPCMV) model. The objectives of this study were: 1) to assess in guinea pigs the protective efficacy against congenital infection and disease of a recombinant live, attenuated vaccine with a targeted deletion of the GPCMV homolog of the HCMV pUL83 tegument protein, GP83; and, 2) to compare the extent of placental infection in vaccine and control groups, using an in situ hybridization (ISH) assay.
Materials and methods: Outbred Hartley guinea pigs were vaccinated prior to pregnancy with a two-dose series of 5×104 pfu of vAM409, a GP83 deletion virus. Deletion of the GP83 gene resulted in an attenuated virus, and vAM409 vaccinated animals did not demonstrate evidence of DNAemia following vaccination, although ELISA antibody responses were comparable to those observed in natural infection. After mating, pregnant animals were challenged with salivary gland-adapted (SG) GPCMV (1×106 pfu) in the second trimester, and pregnancy outcomes were compared to controls.
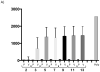
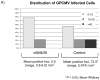
Results: Compared to placebo-immunized controls, vaccination resulted in significantly reduced maternal DNAemia following SG challenge, and there was significantly decreased pup mortality in litters born to vaccinated dams (3/29; 10%), compared to control (35/50; 70%; p<0.001). By in situ hybridization study, recovered placentas in the vAM409 vaccine group demonstrated reduced infection and fewer infectious foci compared to the control group.
Conclusions: In summary, preconception immunization with a GP83 deletion vaccine reduced maternal DNAemia and results in protection against congenital GPCMV-associated pup mortality compared to unvaccinated controls. Vaccination resulted in reduced placental infection, probably related to the reduction in maternal DNAemia. Although the pp65 homolog in GPCMV, GP83, is a known target of protective T cell immune responses, it is nevertheless dispensable for effective vaccination against maternal and fetal CMV disease in this model.
Keywords: CMV UL83; CMV pp65; Cytomegalovirus; Cytomegalovirus vaccine; GPCMV GP83; Guinea pig challenge model; Guinea pig cytomegalovirus; Live; Vaccine efficacy; attenuated CMV vaccine; cytomegalovirus immune evasion.
Figures


Similar articles
-
Comparison of monovalent glycoprotein B with bivalent gB/pp65 (GP83) vaccine for congenital cytomegalovirus infection in a guinea pig model: Inclusion of GP83 reduces gB antibody response but both vaccine approaches provide equivalent protection against pup mortality.Vaccine. 2015 Jul 31;33(32):4013-8. doi: 10.1016/j.vaccine.2015.06.019. Epub 2015 Jun 13. Vaccine. 2015. PMID: 26079615 Free PMC article.
-
Vaccination with a Live Attenuated Cytomegalovirus Devoid of a Protein Kinase R Inhibitory Gene Results in Reduced Maternal Viremia and Improved Pregnancy Outcome in a Guinea Pig Congenital Infection Model.J Virol. 2015 Oct;89(19):9727-38. doi: 10.1128/JVI.01419-15. Epub 2015 Jul 15. J Virol. 2015. PMID: 26178990 Free PMC article.
-
Guinea pig cytomegalovirus protective T cell antigen GP83 is a functional pp65 homolog for innate immune evasion and pentamer dependent virus tropism.J Virol. 2021 Apr 26;95(10):e00324-21. doi: 10.1128/JVI.00324-21. Epub 2021 Mar 3. J Virol. 2021. PMID: 33658350 Free PMC article.
-
The guinea pig as a relevant preclinical model in the rat race for a vaccine against congenital cytomegalovirus infection.Virology. 2025 Sep;610:110560. doi: 10.1016/j.virol.2025.110560. Epub 2025 May 6. Virology. 2025. PMID: 40413831 Review.
-
Comparison of vaccine strategies against congenital CMV infection in the guinea pig model.J Clin Virol. 2008 Mar;41(3):224-30. doi: 10.1016/j.jcv.2007.10.008. Epub 2007 Dec 3. J Clin Virol. 2008. PMID: 18060834 Review.
Cited by
-
A Novel Non-Replication-Competent Cytomegalovirus Capsid Mutant Vaccine Strategy Is Effective in Reducing Congenital Infection.J Virol. 2016 Aug 12;90(17):7902-19. doi: 10.1128/JVI.00283-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334585 Free PMC article.
-
In Vitro Characterization of Human Cytomegalovirus-Targeting Therapeutic Monoclonal Antibodies LJP538 and LJP539.Antimicrob Agents Chemother. 2016 Jul 22;60(8):4961-71. doi: 10.1128/AAC.00382-16. Print 2016 Aug. Antimicrob Agents Chemother. 2016. PMID: 27270290 Free PMC article.
-
Inclusion of the Viral Pentamer Complex in a Vaccine Design Greatly Improves Protection against Congenital Cytomegalovirus in the Guinea Pig Model.J Virol. 2019 Oct 29;93(22):e01442-19. doi: 10.1128/JVI.01442-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31484753 Free PMC article.
-
Distribution and effects of amino acid changes in drug-resistant α and β herpesviruses DNA polymerase.Nucleic Acids Res. 2016 Nov 16;44(20):9530-9554. doi: 10.1093/nar/gkw875. Epub 2016 Sep 29. Nucleic Acids Res. 2016. PMID: 27694307 Free PMC article. Review.
-
Maternal vaccination: moving the science forward.Hum Reprod Update. 2015 Jan-Feb;21(1):119-35. doi: 10.1093/humupd/dmu041. Epub 2014 Jul 11. Hum Reprod Update. 2015. PMID: 25015234 Free PMC article. Review.
References
-
- Whitley RJ. Congenital cytomegalovirus infection: epidemiology and treatment. Adv Exp Med Biol. 2004;549:155–160. - PubMed
-
- Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1992;90(6):862–866. - PubMed
-
- Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(2):233–239. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
