Induction of endoplasmic reticulum-derived replication-competent membrane structures by West Nile virus non-structural protein 4B
- PMID: 24465392
- PMCID: PMC3896337
- DOI: 10.1371/journal.pone.0084040
Induction of endoplasmic reticulum-derived replication-competent membrane structures by West Nile virus non-structural protein 4B
Abstract
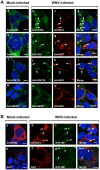
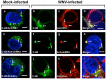
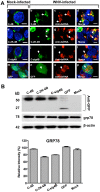
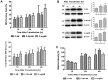
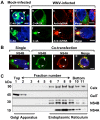
Replication of flaviviruses (family Flaviviridae) occurs in specialized virus-induced membrane structures (IMS). The cellular composition of these IMS varies for different flaviviruses implying different organelle origins for IMS biogenesis. The role of flavivirus non-structural (NS) proteins for the alteration of IMS remains controversial. In this report, we demonstrate that West Nile virus strain New York 99 (WNVNY99) remodels the endoplasmic reticulum (ER) membrane to generate specialized IMS. Within these structures, we observed an element of the cis-Golgi, viral double-stranded RNA, and viral-envelope, NS1, NS4A and NS4B proteins using confocal immunofluorescence microscopy. Biochemical analysis and microscopy revealed that NS4B lacking the 2K-signal peptide associates with the ER membrane where it initiates IMS formation in WNV-infected cells. Co-transfection studies indicated that NS4A and NS4B always remain co-localized in the IMS and are associated with the same membrane fractions, suggesting that these proteins function cooperatively in virus replication and may be an ideal target for antiviral drug discovery.
Conflict of interest statement
Figures


Similar articles
-
Dengue and Zika virus NS4B proteins differ in topology and in determinants of ER membrane protein complex dependency.J Virol. 2025 Feb 25;99(2):e0144324. doi: 10.1128/jvi.01443-24. Epub 2024 Dec 31. J Virol. 2025. PMID: 39745435 Free PMC article.
-
Regulated cleavages at the West Nile virus NS4A-2K-NS4B junctions play a major role in rearranging cytoplasmic membranes and Golgi trafficking of the NS4A protein.J Virol. 2006 May;80(9):4623-32. doi: 10.1128/JVI.80.9.4623-4632.2006. J Virol. 2006. PMID: 16611922 Free PMC article.
-
Protein-protein interactions among West Nile non-structural proteins and transmembrane complex formation in mammalian cells.Virology. 2013 Nov;446(1-2):365-77. doi: 10.1016/j.virol.2013.08.006. Epub 2013 Sep 13. Virology. 2013. PMID: 24074601
-
Dengue Virus Non-Structural Protein 5.Viruses. 2017 Apr 24;9(4):91. doi: 10.3390/v9040091. Viruses. 2017. PMID: 28441781 Free PMC article. Review.
-
Replication cycle and molecular biology of the West Nile virus.Viruses. 2013 Dec 27;6(1):13-53. doi: 10.3390/v6010013. Viruses. 2013. PMID: 24378320 Free PMC article. Review.
Cited by
-
A novel interaction between dengue virus nonstructural protein 1 and the NS4A-2K-4B precursor is required for viral RNA replication but not for formation of the membranous replication organelle.PLoS Pathog. 2019 May 9;15(5):e1007736. doi: 10.1371/journal.ppat.1007736. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31071189 Free PMC article.
-
Tick-Borne Encephalitis Virus: A Structural View.Viruses. 2018 Jun 28;10(7):350. doi: 10.3390/v10070350. Viruses. 2018. PMID: 29958443 Free PMC article. Review.
-
Yeast for virus research.Microb Cell. 2017 Sep 18;4(10):311-330. doi: 10.15698/mic2017.10.592. Microb Cell. 2017. PMID: 29082230 Free PMC article. Review.
-
Functional Roles and Host Interactions of Orthoflavivirus Non-Structural Proteins During Replication.Pathogens. 2025 Feb 12;14(2):184. doi: 10.3390/pathogens14020184. Pathogens. 2025. PMID: 40005559 Free PMC article. Review.
-
Lipidomic Analysis Reveals Serum Alteration of Plasmalogens in Patients Infected With ZIKA Virus.Front Microbiol. 2019 Apr 12;10:753. doi: 10.3389/fmicb.2019.00753. eCollection 2019. Front Microbiol. 2019. PMID: 31031729 Free PMC article.
References
-
- Brinton MA (2002) The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol 56: 371–402. - PubMed
-
- Westaway EG, Khromykh AA, Kenney MT, Mackenzie JM, Jones MK (1997) Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virology 234: 31–41. - PubMed
-
- Miller S, Sparacio S, Bartenschlager R (2006) Subcellular localization and membrane topology of the Dengue virus type 2 Non-structural protein 4B. J Biol Chem 281: 8854–8863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

