Role of macrophages in the altered epithelial function during a type 2 immune response induced by enteric nematode infection
- PMID: 24465430
- PMCID: PMC3900397
- DOI: 10.1371/journal.pone.0084763
Role of macrophages in the altered epithelial function during a type 2 immune response induced by enteric nematode infection
Abstract
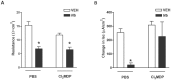
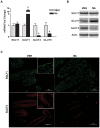
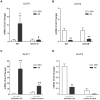
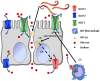
Parasitic enteric nematodes induce a type 2 immune response characterized by increased production of Th2 cytokines, IL-4 and IL-13, and recruitment of alternatively activated macrophages (M2) to the site of infection. Nematode infection is associated with changes in epithelial permeability and inhibition of sodium-linked glucose absorption, but the role of M2 in these effects is unknown. Clodronate-containing liposomes were administered prior to and during nematode infection to deplete macrophages and prevent the development of M2 in response to infection with Nippostrongylus brasiliensis. The inhibition of epithelial glucose absorption that is associated with nematode infection involved a macrophage-dependent reduction in SGLT1 activity, with no change in receptor expression, and a macrophage-independent down-regulation of GLUT2 expression. The reduced transport of glucose into the enterocyte is compensated partially by an up-regulation of the constitutive GLUT1 transporter consistent with stress-induced activation of HIF-1α. Thus, nematode infection results in a "lean" epithelial phenotype that features decreased SGLT1 activity, decreased expression of GLUT2 and an emergent dependence on GLUT1 for glucose uptake into the enterocyte. Macrophages do not play a role in enteric nematode infection-induced changes in epithelial barrier function. There is a greater contribution, however, of paracellular absorption of glucose to supply the energy demands of host resistance. These data provide further evidence of the ability of macrophages to alter glucose metabolism of neighboring cells.
Conflict of interest statement
Figures
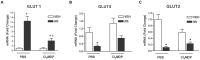
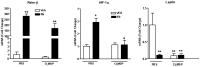
Similar articles
-
Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages.Gastroenterology. 2008 Jul;135(1):217-225.e1. doi: 10.1053/j.gastro.2008.03.077. Epub 2008 Apr 4. Gastroenterology. 2008. PMID: 18471439 Free PMC article.
-
Type 3 muscarinic receptors contribute to intestinal mucosal homeostasis and clearance of Nippostrongylus brasiliensis through induction of TH2 cytokines.Am J Physiol Gastrointest Liver Physiol. 2016 Jul 1;311(1):G130-41. doi: 10.1152/ajpgi.00461.2014. Epub 2016 May 12. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27173511 Free PMC article.
-
Alterations in hexose, amino acid and peptide transporter expression in intestinal epithelial cells during Nippostrongylus brasiliensis infection in the rat.Int J Parasitol. 2003 Oct;33(12):1419-26. doi: 10.1016/s0020-7519(03)00183-8. Int J Parasitol. 2003. PMID: 14527524
-
Critical role of IL-25 in nematode infection-induced alterations in intestinal function.J Immunol. 2010 Dec 1;185(11):6921-9. doi: 10.4049/jimmunol.1000450. Epub 2010 Oct 25. J Immunol. 2010. PMID: 20974983 Free PMC article.
-
Immune polarization by hookworms: taking cues from T helper type 2, type 2 innate lymphoid cells and alternatively activated macrophages.Immunology. 2016 Jun;148(2):115-24. doi: 10.1111/imm.12601. Epub 2016 Mar 31. Immunology. 2016. PMID: 26928141 Free PMC article. Review.
Cited by
-
The Impact of Helminth Coinfection on Innate and Adaptive Immune Resistance and Disease Tolerance during Toxoplasmosis.J Immunol. 2022 Dec 1;209(11):2160-2171. doi: 10.4049/jimmunol.2200504. J Immunol. 2022. PMID: 36426972 Free PMC article.
-
Cryptosporidium parvum alters glucose transport mechanisms in infected enterocytes.Parasitol Res. 2019 Dec;118(12):3429-3441. doi: 10.1007/s00436-019-06471-y. Epub 2019 Oct 31. Parasitol Res. 2019. PMID: 31667591
-
Eosinophils and Macrophages within the Th2-Induced Granuloma: Balancing Killing and Healing in a Tight Space.Infect Immun. 2019 Sep 19;87(10):e00127-19. doi: 10.1128/IAI.00127-19. Print 2019 Oct. Infect Immun. 2019. PMID: 31285249 Free PMC article. Review.
-
Opisthorchis viverrini excretory-secretory products suppress GLUT8 of cholangiocytes.Parasitol Res. 2024 Mar 16;123(3):161. doi: 10.1007/s00436-024-08184-3. Parasitol Res. 2024. PMID: 38491300
-
Regulation of type 2 diabetes by helminth-induced Th2 immune response.J Vet Med Sci. 2017 Jan 10;78(12):1855-1864. doi: 10.1292/jvms.16-0183. Epub 2016 Sep 22. J Vet Med Sci. 2017. PMID: 27665994 Free PMC article.
References
-
- Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, et al. (2004) Helminth parasites–masters of regulation. Immunol Rev 201: 89–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

