Randomised pharmacokinetic trial of rifabutin with lopinavir/ritonavir-antiretroviral therapy in patients with HIV-associated tuberculosis in Vietnam
- PMID: 24465443
- PMCID: PMC3898920
- DOI: 10.1371/journal.pone.0084866
Randomised pharmacokinetic trial of rifabutin with lopinavir/ritonavir-antiretroviral therapy in patients with HIV-associated tuberculosis in Vietnam
Abstract
Background: Rifampicin and protease inhibitors are difficult to use concomitantly in patients with HIV-associated tuberculosis because of drug-drug interactions. Rifabutin has been proposed as an alternative rifamycin, but there is concern that the current recommended dose is suboptimal. The principal aim of this study was to compare bioavailability of two doses of rifabutin (150 mg three times per week and 150 mg daily) in patients with HIV-associated tuberculosis who initiated lopinavir/ritonavir-based antiretroviral therapy in Vietnam. Concentrations of lopinavir/ritonavir were also measured.
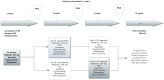
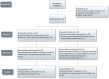
Methods: This was a randomized, open-label, multi-dose, two-arm, cross-over trial, conducted in Vietnamese adults with HIV-associated tuberculosis in Ho Chi Minh City (Clinical trial registry number NCT00651066). Rifabutin pharmacokinetics were evaluated before and after the introduction of lopinavir/ritonavir -based antiretroviral therapy using patient randomization lists. Serial rifabutin and 25-O-desacetyl rifabutin concentrations were measured during a dose interval after 2 weeks of rifabutin 300 mg daily, after 3 weeks of rifabutin 150 mg daily with lopinavir/ritonavir and after 3 weeks of rifabutin 150 mg three times per week with lopinavir/ritonavir.
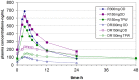
Results: Sixteen and seventeen patients were respectively randomized to the two arms, and pharmacokinetic analysis carried out in 12 and 13 respectively. Rifabutin 150 mg daily with lopinavir/ritonavir was associated with a 32% mean increase in rifabutin average steady state concentration compared with rifabutin 300 mg alone. In contrast, the rifabutin average steady state concentration decreased by 44% when rifabutin was given at 150 mg three times per week with lopinavir/ritonavir. With both dosing regimens, 2 - 5 fold increases of the 25-O-desacetyl- rifabutin metabolite were observed when rifabutin was given with lopinavir/ritonavir compared with rifabutin alone. The different doses of rifabutin had no significant effect on lopinavir/ritonavir plasma concentrations.
Conclusions: Based on these findings, rifabutin 150 mg daily may be preferred when co-administered with lopinavir/ritonavir in patients with HIV-associated tuberculosis.
Trial registration: ClinicalTrials.gov NCT00651066.
Conflict of interest statement
Figures
Similar articles
-
Randomized pharmacokinetic evaluation of different rifabutin doses in African HIV- infected tuberculosis patients on lopinavir/ritonavir-based antiretroviral therapy.BMC Pharmacol Toxicol. 2014 Nov 19;15:61. doi: 10.1186/2050-6511-15-61. BMC Pharmacol Toxicol. 2014. PMID: 25406657 Free PMC article. Clinical Trial.
-
Pharmacokinetic evaluation of rifabutin in combination with lopinavir-ritonavir in patients with HIV infection and active tuberculosis.Clin Infect Dis. 2009 Nov 1;49(9):1305-11. doi: 10.1086/606056. Clin Infect Dis. 2009. PMID: 19807276 Clinical Trial.
-
Pharmacokinetic study of two different rifabutin doses co-administered with lopinavir/ritonavir in African HIV and tuberculosis co-infected adult patients.BMC Infect Dis. 2020 Jun 26;20(1):449. doi: 10.1186/s12879-020-05169-2. BMC Infect Dis. 2020. PMID: 32590942 Free PMC article.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
Protease inhibitor-containing antiretroviral treatment and tuberculosis: can rifabutin fill the breach?Int J Tuberc Lung Dis. 2012 Jan;16(1):6-15. doi: 10.5588/ijtld.10.0626. Epub 2011 Aug 3. Int J Tuberc Lung Dis. 2012. PMID: 21819645 Review.
Cited by
-
Treatment optimization in patients co-infected with HIV and Mycobacterium tuberculosis infections: focus on drug-drug interactions with rifamycins.Clin Pharmacokinet. 2014 Jun;53(6):489-507. doi: 10.1007/s40262-014-0144-3. Clin Pharmacokinet. 2014. PMID: 24777631 Review.
-
Understanding pharmacokinetics to improve tuberculosis treatment outcome.Expert Opin Drug Metab Toxicol. 2014 Jun;10(6):813-23. doi: 10.1517/17425255.2014.895813. Epub 2014 Mar 6. Expert Opin Drug Metab Toxicol. 2014. PMID: 24597717 Free PMC article. Review.
-
Pharmacology, Dosing, and Side Effects of Rifabutin as a Possible Therapy for Antibiotic-Resistant Acinetobacter Infections.Open Forum Infect Dis. 2020 Oct 15;7(11):ofaa460. doi: 10.1093/ofid/ofaa460. eCollection 2020 Nov. Open Forum Infect Dis. 2020. PMID: 33204754 Free PMC article. Review.
-
Population pharmacokinetic drug-drug interaction pooled analysis of existing data for rifabutin and HIV PIs.J Antimicrob Chemother. 2016 May;71(5):1330-40. doi: 10.1093/jac/dkv470. Epub 2016 Jan 31. J Antimicrob Chemother. 2016. PMID: 26832753 Free PMC article.
-
Mycobacterium tuberculosis: 2014 Clinical trials in review.Can J Infect Dis Med Microbiol. 2015 Jan-Feb;26(1):11-4. doi: 10.1155/2015/984635. Can J Infect Dis Med Microbiol. 2015. PMID: 25798148 Free PMC article. No abstract available.
References
-
- UNAIDS (2012) Global Report. UNAIDS Report on the Global AIDS Epidemic.
-
- World Health Organization (2012) Global Tuberculosis Report. WHO, Geneva Switzerland. WHO/HTM/TB/2012.6.
-
- World Health Organization, UNAIDS and UNICEF (2011) Global HIV/AIDS Response. Epidemic update and health sector progress towards Universal Access. Progress Report 2011. WHO, Geneva, Switzerland.
-
- UNAIDS and WHO (2012) The Treatment 2.0 Framework for Action: Catalysing the next phase of treatment, care and support.Geneva, Switzerland.
-
- World Health Organization (2013) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. June 2013. Geneva, Switzerland. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

