Surfactant secretion in LRRK2 knock-out rats: changes in lamellar body morphology and rate of exocytosis
- PMID: 24465451
- PMCID: PMC3897396
- DOI: 10.1371/journal.pone.0084926
Surfactant secretion in LRRK2 knock-out rats: changes in lamellar body morphology and rate of exocytosis
Abstract
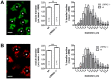
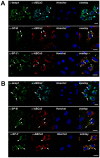
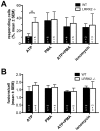
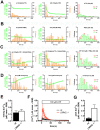
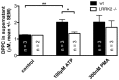
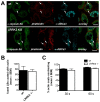
Leucine-rich repeat kinase 2 (LRRK2) is known to play a role in the pathogenesis of various diseases including Parkinson disease, morbus Crohn, leprosy and cancer. LRRK2 is suggested to be involved in a number of cell biological processes such as vesicular trafficking, transcription, autophagy and lysosomal pathways. Recent histological studies of lungs of LRRK2 knock-out (LRRK2 -/-) mice revealed significantly enlarged lamellar bodies (LBs) in alveolar type II (ATII) epithelial cells. LBs are large, lysosome-related storage organelles for pulmonary surfactant, which is released into the alveolar lumen upon LB exocytosis. In this study we used high-resolution, subcellular live-cell imaging assays to investigate whether similar morphological changes can be observed in primary ATII cells from LRRK2 -/- rats and whether such changes result in altered LB exocytosis. Similarly to the report in mice, ATII cells from LRRK2 -/- rats contained significantly enlarged LBs resulting in a >50% increase in LB volume. Stimulation of ATII cells with ATP elicited LB exocytosis in a significantly increased proportion of cells from LRRK2 -/- animals. LRRK2 -/- cells also displayed increased intracellular Ca(2+) release upon ATP treatment and significant triggering of LB exocytosis. These findings are in line with the strong Ca(2+)-dependence of LB fusion activity and suggest that LRRK2 -/- affects exocytic response in ATII cells via modulating intracellular Ca(2+) signaling. Post-fusion regulation of surfactant secretion was unaltered. Actin coating of fused vesicles and subsequent vesicle compression to promote surfactant expulsion were comparable in cells from LRRK2 -/- and wt animals. Surprisingly, surfactant (phospholipid) release from LRRK2 -/- cells was reduced following stimulation of LB exocytosis possibly due to impaired LB maturation and surfactant loading of LBs. In summary our results suggest that LRRK2 -/- affects LB size, modulates intracellular Ca(2+) signaling and promotes LB exocytosis upon stimulation of ATII cells with ATP.
Conflict of interest statement
Figures
Similar articles
-
Fusion-activated cation entry (FACE) via P2X₄ couples surfactant secretion and alveolar fluid transport.FASEB J. 2013 Apr;27(4):1772-83. doi: 10.1096/fj.12-220533. Epub 2013 Jan 10. FASEB J. 2013. PMID: 23307836
-
Microfluidic shear stress-regulated surfactant secretion in alveolar epithelial type II cells in vitro.Am J Physiol Lung Cell Mol Physiol. 2014 Apr 1;306(7):L672-83. doi: 10.1152/ajplung.00106.2013. Epub 2014 Jan 31. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24487389
-
Actin coating and compression of fused secretory vesicles are essential for surfactant secretion--a role for Rho, formins and myosin II.J Cell Sci. 2012 Jun 1;125(Pt 11):2765-74. doi: 10.1242/jcs.105262. Epub 2012 Mar 16. J Cell Sci. 2012. PMID: 22427691
-
The conception of fusion pores as rate-limiting structures for surfactant secretion.Comp Biochem Physiol A Mol Integr Physiol. 2001 May;129(1):227-31. doi: 10.1016/s1095-6433(01)00319-1. Comp Biochem Physiol A Mol Integr Physiol. 2001. PMID: 11369547 Review.
-
Lamellar body exocytosis by cell stretch or purinergic stimulation: possible physiological roles, messengers and mechanisms.Cell Physiol Biochem. 2010;25(1):1-12. doi: 10.1159/000272046. Epub 2009 Dec 22. Cell Physiol Biochem. 2010. PMID: 20054140 Review.
Cited by
-
Cellular processes associated with LRRK2 function and dysfunction.FEBS J. 2015 Aug;282(15):2806-26. doi: 10.1111/febs.13305. Epub 2015 May 9. FEBS J. 2015. PMID: 25899482 Free PMC article. Review.
-
Glial phagocytic clearance in Parkinson's disease.Mol Neurodegener. 2019 Apr 5;14(1):16. doi: 10.1186/s13024-019-0314-8. Mol Neurodegener. 2019. PMID: 30953527 Free PMC article. Review.
-
SNAREs in the maturation and function of LROs.Bioarchitecture. 2016;6(1):1-11. doi: 10.1080/19490992.2015.1131890. Bioarchitecture. 2016. PMID: 26760525 Free PMC article. Review.
-
Parkinson's disease genes VPS35 and EIF4G1 interact genetically and converge on α-synuclein.Neuron. 2015 Jan 7;85(1):76-87. doi: 10.1016/j.neuron.2014.11.027. Epub 2014 Dec 18. Neuron. 2015. PMID: 25533483 Free PMC article.
-
Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles.J Cell Sci. 2015 Mar 15;128(6):1193-203. doi: 10.1242/jcs.165571. Epub 2015 Jan 30. J Cell Sci. 2015. PMID: 25637593 Free PMC article.
References
-
- Bosgraaf L, Van Haastert PJ (2003) Roc, a Ras/GTPase domain in complex proteins. BiochimBiophysActa 1643: 5–10. - PubMed
-
- Drolet RE, Sanders JM, Kern JT (2011) Leucine-rich repeat kinase 2 (LRRK2) cellular biology: a review of recent advances in identifying physiological substrates and cellular functions. J Neurogenet 25: 140–151. - PubMed
-
- Berwick DC, Harvey K (2011) LRRK2 signaling pathways: the key to unlocking neurodegeneration? Trends Cell Biol 21: 257–265. - PubMed
-
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, et al. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44: 595–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

