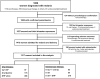
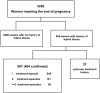
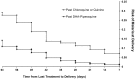
Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy
- PMID: 24465458
- PMCID: PMC3894943
- DOI: 10.1371/journal.pone.0084976
Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy
Abstract
Background: Artemisinin combination therapy (ACT) is recommended for the treatment of multidrug resistant malaria in the second and third trimesters of pregnancy, but the experience with ACTs is limited. We review the exposure of pregnant women to the combination dihydroartemisinin-piperaquine over a 6 year period.
Methods: From April 2004-June 2009, a prospective hospital-based surveillance screened all pregnant women for malaria and documented maternal and neonatal outcomes.
Results: Data were available on 6519 pregnant women admitted to hospital; 332 (5.1%) women presented in the first trimester, 324 (5.0%) in the second, 5843 (89.6%) in the third, and in 20 women the trimester was undocumented. Peripheral parasitaemia was confirmed in 1682 women, of whom 106 (6.3%) had severe malaria. Of the 1217 women admitted with malaria in the second and third trimesters without an impending adverse outcome, those treated with DHP were more likely to be discharged with an ongoing pregnancy compared to those treated with a non-ACT regimen (Odds Ratio OR = 2.48 [1.26-4.86]); p = 0.006. However in the first trimester 63% (5/8) of women treated with oral DHP miscarried compared to 2.6% (1/38) of those receiving oral quinine; p<0.001. Of the 847 women admitted for delivery those reporting a history of malaria during their pregnancy who had been treated with quinine-based regimens rather than DHP had a higher risk of malaria at delivery (adjusted OR = 1.56 (95%CI 0.97-2.5), p = 0.068) and perinatal mortality (adjusted OR = 3.17 [95%CI: 1.17-8.60]; p = 0.023).
Conclusions: In the second and third trimesters of pregnancy, a three day course of DHP simplified antimalarial treatment and had significant benefits over quinine-based regimens in reducing recurrent malaria and poor fetal outcome. These data provide reassuring evidence for the rational design of prospective randomized clinical trials and pharmacokinetic studies.
Conflict of interest statement
Figures
References
-
- Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, et al. (2007) Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 7: 93–104. - PubMed
-
- Brabin BJ, Hakimi M, Pelletier D (2001) An analysis of anemia and pregnancy-related maternal mortality. J Nutr 131: 604S–614S discussion 614S–615S. - PubMed
-
- WHO/AFRO (2004) A strategic framework for malaria prevention and control during pregnancy in the African region. World Health Organization Regional Office for Africa
-
- Nosten F, McGready R, Mutabingwa T (2007) Case management of malaria in pregnancy. Lancet Infect Dis 7: 118–125. - PubMed
-
- Rijken MJ, McGready R, Boel ME, Poespoprodjo R, Singh N, et al. (2012) Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis 12: 75–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous