mUbiSiDa: a comprehensive database for protein ubiquitination sites in mammals
- PMID: 24465676
- PMCID: PMC3894998
- DOI: 10.1371/journal.pone.0085744
mUbiSiDa: a comprehensive database for protein ubiquitination sites in mammals
Abstract
Motivation: Protein ubiquitination is one of the important post-translational modifications by attaching ubiquitin to specific lysine (K) residues in target proteins, and plays important regulatory roles in many cell processes. Recent studies indicated that abnormal protein ubiquitination have been implicated in many diseases by degradation of many key regulatory proteins including tumor suppressor, oncoprotein, and cell cycle regulator. The detailed information of protein ubiquitination sites is useful for scientists to investigate the mechanism of many cell activities and related diseases.
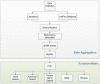
Results: In this study we established mUbiSida for mammalian Ubiquitination Site Database, which provides a scientific community with a comprehensive, freely and high-quality accessible resource of mammalian protein ubiquitination sites. In mUbiSida, we deposited about 35,494 experimentally validated ubiquitinated proteins with 110,976 ubiquitination sites from five species. The mUbiSiDa can also provide blast function to predict novel protein ubiquitination sites in other species by blast the query sequence in the deposit sequences in mUbiSiDa. The mUbiSiDa was designed to be a widely used tool for biologists and biomedical researchers with a user-friendly interface, and facilitate the further research of protein ubiquitination, biological networks and functional proteomics. The mUbiSiDa database is freely available at http://reprod.njmu.edu.cn/mUbiSiDa.
Conflict of interest statement
Figures
References
-
- Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67: 425–479. - PubMed
-
- Hershko A (2005) The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ 12: 1191–1197. - PubMed
-
- Pickart CM, Eddins MJ (2004) Ubiquitin: structures, functions, mechanisms. Biochim. Biophys Acta 1695: 55–72. - PubMed
-
- Sorokin AV, Kim ER, Ovchinnikov LP (2009) Proteasome system of protein degradation and processing. Biochemistry 74(13): 1411–1442. - PubMed
-
- Walsh CT, Garneau TS, Gatto GJ Jr (2005) Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl 44: 7342–7372. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials