Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering
- PMID: 24465736
- PMCID: PMC3897540
- DOI: 10.1371/journal.pone.0085835
Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering
Abstract
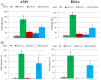
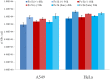
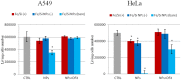
We have studied in vitro toxicity of iron oxide nanoparticles (NPs) coated with a thin silica shell (Fe3O4/SiO2 NPs) on A549 and HeLa cells. We compared bare and surface passivated Fe3O4/SiO2 NPs to evaluate the effects of the coating on the particle stability and toxicity. NPs cytotoxicity was investigated by cell viability, membrane integrity, mitochondrial membrane potential (MMP), reactive oxygen species (ROS) assays, and their genotoxicity by comet assay. Our results show that NPs surface passivation reduces the oxidative stress and alteration of iron homeostasis and, consequently, the overall toxicity, despite bare and passivated NPs show similar cell internalization efficiency. We found that the higher toxicity of bare NPs is due to their stronger in-situ degradation, with larger intracellular release of iron ions, as compared to surface passivated NPs. Our results indicate that surface engineering of Fe3O4/SiO2 NPs plays a key role in improving particles stability in biological environments reducing both cytotoxic and genotoxic effects.
Conflict of interest statement
Figures

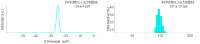





Similar articles
-
Dye-doped biodegradable nanoparticle SiO2 coating on zinc- and iron-oxide nanoparticles to improve biocompatibility and for in vivo imaging studies.Nanoscale. 2020 Mar 14;12(10):6164-6175. doi: 10.1039/c9nr08743e. Epub 2020 Mar 5. Nanoscale. 2020. PMID: 32133463
-
Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro.Hum Exp Toxicol. 2016 Feb;35(2):170-83. doi: 10.1177/0960327115579208. Epub 2015 Mar 31. Hum Exp Toxicol. 2016. PMID: 25829403
-
Cytotoxicity and oxidative stress responses of silica-coated iron oxide nanoparticles in CHSE-214 cells.Environ Sci Pollut Res Int. 2017 Jan;24(2):2055-2064. doi: 10.1007/s11356-016-7870-z. Epub 2016 Nov 3. Environ Sci Pollut Res Int. 2017. PMID: 27807789
-
Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues.Artif Cells Nanomed Biotechnol. 2020 Dec;48(1):443-451. doi: 10.1080/21691401.2019.1709855. Artif Cells Nanomed Biotechnol. 2020. PMID: 32024389 Review.
-
Evaluating the toxicity of selected types of nanochemicals.Rev Environ Contam Toxicol. 2012;215:39-121. doi: 10.1007/978-1-4614-1463-6_2. Rev Environ Contam Toxicol. 2012. PMID: 22057930 Review.
Cited by
-
In vitro toxicity evaluation of silica-coated iron oxide nanoparticles in human SHSY5Y neuronal cells.Toxicol Res (Camb). 2015 Oct 23;5(1):235-247. doi: 10.1039/c5tx00206k. eCollection 2016 Jan 1. Toxicol Res (Camb). 2015. PMID: 30090340 Free PMC article.
-
Effects of PEGylated Fe-Fe3O4 core-shell nanoparticles on NIH3T3 and A549 cell lines.Heliyon. 2019 Dec 27;6(1):e03124. doi: 10.1016/j.heliyon.2019.e03124. eCollection 2020 Jan. Heliyon. 2019. PMID: 31909281 Free PMC article.
-
Magnetic nanoparticles and nanocomposites for remote controlled therapies.J Control Release. 2015 Dec 10;219:76-94. doi: 10.1016/j.jconrel.2015.09.039. Epub 2015 Sep 25. J Control Release. 2015. PMID: 26407670 Free PMC article. Review.
-
Manganese-Zinc Ferrites: Safe and Efficient Nanolabels for Cell Imaging and Tracking In Vivo.ChemistryOpen. 2019 Jan 23;8(2):155-165. doi: 10.1002/open.201800261. eCollection 2019 Feb. ChemistryOpen. 2019. PMID: 30740290 Free PMC article.
-
Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation.BMC Neurosci. 2017 Jun 26;18(1):51. doi: 10.1186/s12868-017-0369-9. BMC Neurosci. 2017. PMID: 28651647 Free PMC article. Review.
References
-
- Murruni LG, Solanes V, Debray M, Kreiner AJ, Davidson J, et al. (2009) Concentrations and elemental composition of particulate matter in the Buenos Aires underground system. Atmos Environ 43: 4577–4583.
-
- Lorenzo R, Kaegi R, Gehrig R, Grobety B (2006) Particle emissions of a railway line determined by detailed single particle analysis. Atmos Environ 40: 7831–7841.
-
- Sowards JW, Lippold JC, Dickinson DW, Ramirez AJ (2008) Characterization of Welding Fume from SMAW Electrodes. Weld J 87: 106s–112s.
-
- Na HB, Song IC, Hyeon T (2009) Inorganic nanoparticles for MRI contrast agents. Adv Mater 21: 2133–2148.
-
- Qiao RR, Yang CH, Gao MY (2009) Superparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applications. J Mater Chem 19: 6274–6293.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

