Mechanism of deep-sea fish α-actin pressure tolerance investigated by molecular dynamics simulations
- PMID: 24465747
- PMCID: PMC3896411
- DOI: 10.1371/journal.pone.0085852
Mechanism of deep-sea fish α-actin pressure tolerance investigated by molecular dynamics simulations
Abstract
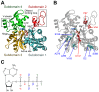
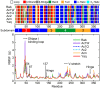
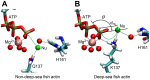
The pressure tolerance of monomeric α-actin proteins from the deep-sea fish Coryphaenoides armatus and C. yaquinae was compared to that of non-deep-sea fish C. acrolepis, carp, and rabbit/human/chicken actins using molecular dynamics simulations at 0.1 and 60 MPa. The amino acid sequences of actins are highly conserved across a variety of species. The actins from C. armatus and C. yaquinae have the specific substitutions Q137K/V54A and Q137K/L67P, respectively, relative to C. acrolepis, and are pressure tolerant to depths of at least 6000 m. At high pressure, we observed significant changes in the salt bridge patterns in deep-sea fish actins, and these changes are expected to stabilize ATP binding and subdomain arrangement. Salt bridges between ATP and K137, formed in deep-sea fish actins, are expected to stabilize ATP binding even at high pressure. At high pressure, deep-sea fish actins also formed a greater total number of salt bridges than non-deep-sea fish actins owing to the formation of inter-helix/strand and inter-subdomain salt bridges. Free energy analysis suggests that deep-sea fish actins are stabilized to a greater degree by the conformational energy decrease associated with pressure effect.
Conflict of interest statement
Figures
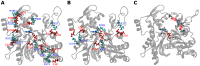
Similar articles
-
High-pressure adaptation of muscle proteins from deep-sea fishes, Coryphaenoides yaquinae and C. armatus.Ann N Y Acad Sci. 2010 Feb;1189:91-4. doi: 10.1111/j.1749-6632.2009.05181.x. Ann N Y Acad Sci. 2010. PMID: 20233373
-
Structure-based analysis of high pressure adaptation of alpha-actin.J Biol Chem. 2003 Jul 25;278(30):28060-6. doi: 10.1074/jbc.M302328200. Epub 2003 May 8. J Biol Chem. 2003. PMID: 12740368
-
Comparative sequence analysis of myosin heavy chain proteins from congeneric shallow- and deep-living rattail fish (genus Coryphaenoides).J Exp Biol. 2008 May;211(Pt 9):1362-7. doi: 10.1242/jeb.017137. J Exp Biol. 2008. PMID: 18424669
-
Environmental adaptation of proteins: strategies for the conservation of critical functional and structural traits.Comp Biochem Physiol A Comp Physiol. 1983;76(3):621-33. doi: 10.1016/0300-9629(83)90464-4. Comp Biochem Physiol A Comp Physiol. 1983. PMID: 6139233 Review.
-
North Atlantic demersal deep-water fish distribution and biology: present knowledge and challenges for the future.J Fish Biol. 2013 Dec;83(6):1489-507. doi: 10.1111/jfb.12208. Epub 2013 Sep 3. J Fish Biol. 2013. PMID: 24298948 Review.
Cited by
-
Convergent Evolution and Structural Adaptation to the Deep Ocean in the Protein-Folding Chaperonin CCTα.Genome Biol Evol. 2020 Nov 3;12(11):1929-1942. doi: 10.1093/gbe/evaa167. Genome Biol Evol. 2020. PMID: 32780796 Free PMC article.
-
Distribution of the Order Lampriformes in the Mediterranean Sea with Notes on Their Biology, Morphology, and Taxonomy.Biology (Basel). 2022 Oct 19;11(10):1534. doi: 10.3390/biology11101534. Biology (Basel). 2022. PMID: 36290437 Free PMC article. Review.
-
Homeocurvature adaptation of phospholipids to pressure in deep-sea invertebrates.Science. 2024 Jun 28;384(6703):1482-1488. doi: 10.1126/science.adm7607. Epub 2024 Jun 27. Science. 2024. PMID: 38935710 Free PMC article.
-
Session 1SHA-control of biological functions with hydrostatic pressure stimulation.Biophys Rev. 2020 Apr;12(2):269-270. doi: 10.1007/s12551-020-00658-9. Epub 2020 Mar 3. Biophys Rev. 2020. PMID: 32125656 Free PMC article. No abstract available.
-
Molecular adaptation to high pressure in cytochrome P450 1A and aryl hydrocarbon receptor systems of the deep-sea fish Coryphaenoides armatus.Biochim Biophys Acta Proteins Proteom. 2018 Jan;1866(1):155-165. doi: 10.1016/j.bbapap.2017.06.026. Epub 2017 Jul 8. Biochim Biophys Acta Proteins Proteom. 2018. PMID: 28694077 Free PMC article.
References
-
- Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465. - PubMed
-
- Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC (1990) Atomic-structure of the actin:DNase-I complex. Nature 347: 37–44. - PubMed
-
- Oda T, Iwasa M, Aihara T, Maeda Y, Narita A (2009) The nature of the globular- to fibrous-actin transition. Nature 457: 441–445. - PubMed
-
- Murakami K, Yasunaga T, Noguchi TQP, Gomibuchi Y, Ngo KX, et al. (2010) Structural basis for actin assembly, activation of ATP hydrolysis, and delayed phosphate release. Cell 143: 275–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

