Dopamine signaling regulates fat content through β-oxidation in Caenorhabditis elegans
- PMID: 24465759
- PMCID: PMC3899111
- DOI: 10.1371/journal.pone.0085874
Dopamine signaling regulates fat content through β-oxidation in Caenorhabditis elegans
Abstract
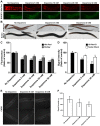
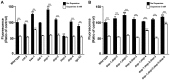
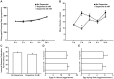
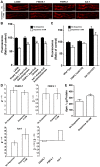
The regulation of energy balance involves an intricate interplay between neural mechanisms that respond to internal and external cues of energy demand and food availability. Compelling data have implicated the neurotransmitter dopamine as an important part of body weight regulation. However, the precise mechanisms through which dopamine regulates energy homeostasis remain poorly understood. Here, we investigate mechanisms through which dopamine modulates energy storage. We showed that dopamine signaling regulates fat reservoirs in Caenorhabditis elegans. We found that the fat reducing effects of dopamine were dependent on dopaminergic receptors and a set of fat oxidation enzymes. Our findings reveal an ancient role for dopaminergic regulation of fat and suggest that dopamine signaling elicits this outcome through cascades that ultimately mobilize peripheral fat depots.
Conflict of interest statement
Figures
References
-
- Gallant P, Malutan T, McLean H, Verellen L, Caveney S, et al.. (2003) Functionally distinct dopamine and octopamine transporters in the CNS of the cabbage looper moth. Eur J Biochem 270: : 664–674. 3417 [pii]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

