Phosphomimetic modulation of eNOS improves myocardial reperfusion and mimics cardiac postconditioning in mice
- PMID: 24465805
- PMCID: PMC3897570
- DOI: 10.1371/journal.pone.0085946
Phosphomimetic modulation of eNOS improves myocardial reperfusion and mimics cardiac postconditioning in mice
Abstract
Objective: Myocardial infarction resulting from ischemia-reperfusion injury can be reduced by cardiac postconditioning, in which blood flow is restored intermittently prior to full reperfusion. Although key molecular mechanisms and prosurvival pathways involved in postconditioning have been identified, a direct role for eNOS-derived NO in improving regional myocardial perfusion has not been shown. The objective of this study is to measure, with high temporal and spatial resolution, regional myocardial perfusion during ischemia-reperfusion and postconditioning, in order to determine the contribution of regional blood flow effects of NO to infarct size and protection.
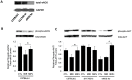
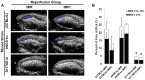
Methods and results: We used myocardial contrast echocardiography to measure regional myocardial blood flow in mice over time. Reperfusion after myocardial ischemia-reperfusion injury is improved by postconditioning, as well as by phosphomimetic eNOS modulation. Knock-in mice expressing a phosphomimetic S1176D form of eNOS showed improved myocardial reperfusion and significantly reduced infarct size. eNOS knock-out mice failed to show cardioprotection from postconditioning. The size of the no-reflow zone following ischemia-reperfusion is substantially reduced by postconditioning and by the phosphomimetic eNOS mutation.
Conclusions and significance: Using myocardial contrast echocardiography, we show that temporal dynamics of regional myocardial perfusion restoration contribute to reduced infarct size after postconditioning. eNOS has direct effects on myocardial blood flow following ischemia-reperfusion, with reduction in the size of the no-reflow zone. These results have important implications for ongoing clinical trials on cardioprotection, because the degree of protective benefit may be significantly influenced by the regional hemodynamic effects of eNOS-derived NO.
Conflict of interest statement
Figures



Similar articles
-
Hydromorphine postconditioning protects isolated rat heart against ischemia-reperfusion injury via activating P13K/Akt/eNOS signaling.Cardiovasc Ther. 2018 Dec;36(6):e12481. doi: 10.1111/1755-5922.12481. Cardiovasc Ther. 2018. PMID: 30597772
-
Remote Limb Ischaemic Postconditioning Protects Against Myocardial Ischaemia/Reperfusion Injury in Mice: Activation of JAK/STAT3-Mediated Nrf2-Antioxidant Signalling.Cell Physiol Biochem. 2017;43(3):1140-1151. doi: 10.1159/000481755. Epub 2017 Oct 5. Cell Physiol Biochem. 2017. PMID: 28977786
-
Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism.Cardiovasc Res. 2017 May 1;113(6):609-619. doi: 10.1093/cvr/cvw246. Cardiovasc Res. 2017. PMID: 28073832
-
Postconditioning--A new link in nature's armor against myocardial ischemia-reperfusion injury.Basic Res Cardiol. 2005 Jul;100(4):295-310. doi: 10.1007/s00395-005-0523-x. Epub 2005 Mar 30. Basic Res Cardiol. 2005. PMID: 15793629 Review.
-
Organ dysfunction following regional and global ischemia/reperfusion. Intervention with postconditioning and adenocaine.Dan Med J. 2012 Aug;59(8):B4496. Dan Med J. 2012. PMID: 22849985 Review.
Cited by
-
Postconditioning with Nitrates Protects Against Myocardial Reperfusion Injury: A New Use for an Old Pharmacological Agent.Med Sci Monit. 2020 Jun 9;26:e923129. doi: 10.12659/MSM.923129. Med Sci Monit. 2020. PMID: 32516304 Free PMC article. Review.
-
Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis.J Pineal Res. 2017 Aug;63(1):e12413. doi: 10.1111/jpi.12413. Epub 2017 Apr 27. J Pineal Res. 2017. PMID: 28398674 Free PMC article.
-
Biomedical Imaging in Experimental Models of Cardiovascular Disease.Circ Res. 2022 Jun 10;130(12):1851-1868. doi: 10.1161/CIRCRESAHA.122.320306. Epub 2022 Jun 9. Circ Res. 2022. PMID: 35679370 Free PMC article. Review.
References
-
- Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, et al. (2004) Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res 62: 74–85. - PubMed
-
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, et al. (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H579–588. - PubMed
-
- Ludman AJ, Yellon DM, Hausenloy DJ (2010) Cardiac preconditioning for ischaemia: lost in translation. Dis Model Mech 3: 35–38. - PubMed
-
- Mewton N, Ivanes F, Cour M, Ovize M (2010) Postconditioning: from experimental proof to clinical concept. Dis Model Mech 3: 39–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

