Structural basis of thermal stability of the tungsten cofactor synthesis protein MoaB from Pyrococcus furiosus
- PMID: 24465852
- PMCID: PMC3896444
- DOI: 10.1371/journal.pone.0086030
Structural basis of thermal stability of the tungsten cofactor synthesis protein MoaB from Pyrococcus furiosus
Abstract
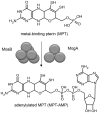
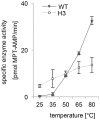
Molybdenum and tungsten cofactors share a similar pterin-based scaffold, which hosts an ene-dithiolate function being essential for the coordination of either molybdenum or tungsten. The biosynthesis of both cofactors involves a multistep pathway, which ends with the activation of the metal binding pterin (MPT) by adenylylation before the respective metal is incorporated. In the hyperthermophilic organism Pyrococcus furiosus, the hexameric protein MoaB (PfuMoaB) has been shown to catalyse MPT-adenylylation. Here we determined the crystal structure of PfuMoaB at 2.5 Å resolution and identified key residues of α3-helix mediating hexamer formation. Given that PfuMoaB homologues from mesophilic organisms form trimers, we investigated the impact on PfuMoaB hexamerization on thermal stability and activity. Using structure-guided mutagenesis, we successfully disrupted the hexamer interface in PfuMoaB. The resulting PfuMoaB-H3 variant formed monomers, dimers and trimers as determined by size exclusion chromatography. Circular dichroism spectroscopy as well as chemical cross-linking coupled to mass spectrometry confirmed a wild-type-like fold of the protomers as well as inter-subunits contacts. The melting temperature of PfuMoaB-H3 was found to be reduced by more than 15 °C as determined by differential scanning calorimetry, thus demonstrating hexamerization as key determinant for PfuMoaB thermal stability. Remarkably, while a loss of activity at temperatures higher than 50 °C was observed in the PfuMoaB-H3 variant, at lower temperatures, we determined a significantly increased catalytic activity. The latter suggests a gain in conformational flexibility caused by the disruption of the hexamerization interface.
Conflict of interest statement
Figures


Similar articles
-
Function of MoaB proteins in the biosynthesis of the molybdenum and tungsten cofactors.Biochemistry. 2008 Jan 22;47(3):949-56. doi: 10.1021/bi7020487. Epub 2007 Dec 22. Biochemistry. 2008. PMID: 18154309
-
Structure of a hyperthermophilic tungstopterin enzyme, aldehyde ferredoxin oxidoreductase.Science. 1995 Mar 10;267(5203):1463-9. doi: 10.1126/science.7878465. Science. 1995. PMID: 7878465
-
Structural basis for oligosaccharide recognition by Pyrococcus furiosus maltodextrin-binding protein.J Mol Biol. 2001 Jan 26;305(4):891-904. doi: 10.1006/jmbi.2000.4202. J Mol Biol. 2001. PMID: 11162100
-
Formaldehyde ferredoxin oxidoreductase from Pyrococcus furiosus: the 1.85 A resolution crystal structure and its mechanistic implications.J Mol Biol. 1999 Feb 26;286(3):899-914. doi: 10.1006/jmbi.1998.2488. J Mol Biol. 1999. PMID: 10024458
-
A monomeric TIM-barrel structure from Pyrococcus furiosus is optimized for extreme temperatures.Acta Crystallogr D Biol Crystallogr. 2012 Nov;68(Pt 11):1479-87. doi: 10.1107/S0907444912037171. Epub 2012 Oct 13. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 23090397
Cited by
-
Development of a univariate membrane-based mid-infrared method for protein quantitation and total lipid content analysis of biological samples.J Anal Methods Chem. 2014;2014:657079. doi: 10.1155/2014/657079. Epub 2014 Oct 13. J Anal Methods Chem. 2014. PMID: 25371845 Free PMC article.
-
The thermophilic biomass-degrading bacterium Caldicellulosiruptor bescii utilizes two enzymes to oxidize glyceraldehyde 3-phosphate during glycolysis.J Biol Chem. 2019 Jun 21;294(25):9995-10005. doi: 10.1074/jbc.RA118.007120. Epub 2019 May 16. J Biol Chem. 2019. PMID: 31097544 Free PMC article.
-
Sequences Flanking the Gephyrin-Binding Site of GlyRβ Tune Receptor Stabilization at Synapses.eNeuro. 2018 Feb 19;5(1):ENEURO.0042-17.2018. doi: 10.1523/ENEURO.0042-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29464196 Free PMC article.
-
MoaB2, a newly identified transcription factor, binds to σA in Mycobacterium smegmatis.J Bacteriol. 2024 Dec 19;206(12):e0006624. doi: 10.1128/jb.00066-24. Epub 2024 Nov 5. J Bacteriol. 2024. PMID: 39499088 Free PMC article.
References
-
- Bevers LE, Hagedoorn P-L, Hagen WR (2009) The bioinorganic chemistry of tungsten. Coordination Chemistry Reviews 253: 269–290 10.1016/j.ccr.2008.01.017 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

