The productive entry pathway of HIV-1 in macrophages is dependent on endocytosis through lipid rafts containing CD4
- PMID: 24465876
- PMCID: PMC3899108
- DOI: 10.1371/journal.pone.0086071
The productive entry pathway of HIV-1 in macrophages is dependent on endocytosis through lipid rafts containing CD4
Abstract
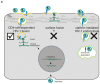
Macrophages constitute an important reservoir of HIV-1 infection, yet HIV-1 entry into these cells is poorly understood due to the difficulty in genetically manipulating primary macrophages. We developed an effective genetic approach to manipulate the sub-cellular distribution of CD4 in macrophages, and investigated how this affects the HIV-1 entry pathway. Pluripotent Stem Cells (PSC) were transduced with lentiviral vectors designed to manipulate CD4 location and were then differentiated into genetically modified macrophages. HIV-1 infection of these cells was assessed by performing assays that measure critical steps of the HIV-1 lifecycle (fusion, reverse transcription, and expression from HIV-1 integrants). Expression of LCK (which tethers CD4 to the surface of T cells, but is not normally expressed in macrophages) in PSC-macrophages effectively tethered CD4 at the cell surface, reducing its normal endocytic recycling route, and increasing surface CD4 expression 3-fold. This led to a significant increase in HIV-1 fusion and reverse transcription, but productive HIV-1 infection efficiency (as determined by reporter expression from DNA integrants) was unaffected. This implies that surface-tethering of CD4 sequesters HIV-1 into a pathway that is unproductive in macrophages. Secondly, to investigate the importance of lipid rafts (as detergent resistant membranes - DRM) in HIV-1 infection, we generated genetically modified PSC-macrophages that express CD4 mutants known to be excluded from DRM. These macrophages were significantly less able to support HIV-1 fusion, reverse-transcription and integration than engineered controls. Overall, these results support a model in which productive infection by HIV-1 in macrophages occurs via a CD4-raft-dependent endocytic uptake pathway.
Conflict of interest statement
Figures






References
-
- Harbison MA, Gillis JM, Pinkston P, Byrn RA, Rose RM, et al. (1990) Effects of recombinant soluble CD4 (rCD4) on HIV-1 infection of monocyte/macrophages. The Journal of infectious diseases 161: 1–6. - PubMed
-
- Collin M, Herbein G, Montaner L, Gordon S (1993) PCR analysis of HIV1 infection of macrophages: virus entry is CD4-dependent. Research in virology 144: 13–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

