Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype
- PMID: 24465881
- PMCID: PMC3897642
- DOI: 10.1371/journal.pone.0086078
Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype
Abstract
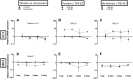
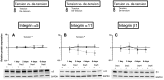
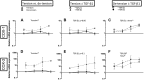
Mechanical loading of tendon cells results in an upregulation of mechanotransduction signaling pathways, cell-matrix adhesion and collagen synthesis, but whether unloading removes these responses is unclear. We investigated the response to tension release, with regard to matrix proteins, pro-inflammatory mediators and tendon phenotypic specific molecules, in an in vitro model where tendon-like tissue was engineered from human tendon cells. Tissue sampling was performed 1, 2, 4 and 6 days after surgical de-tensioning of the tendon construct. When tensile stimulus was removed, integrin type collagen receptors showed a contrasting response with a clear drop in integrin subunit α11 mRNA and protein expression, and an increase in α2 integrin mRNA and protein levels. Further, specific markers for tendon cell differentiation declined and normal tendon architecture was disturbed, whereas pro-inflammatory molecules were upregulated. Stimulation with the cytokine TGF-β1 had distinct effects on some tendon-related genes in both tensioned and de-tensioned tissue. These findings indicate an important role of mechanical loading for cellular and matrix responses in tendon, including that loss of tension leads to a decrease in phenotypical markers for tendon, while expression of pro-inflammatory mediators is induced.
Conflict of interest statement
Figures








Similar articles
-
Tendon-derived extracellular matrix induces mesenchymal stem cell tenogenesis via an integrin/transforming growth factor-β crosstalk-mediated mechanism.FASEB J. 2020 Jun;34(6):8172-8186. doi: 10.1096/fj.201902377RR. Epub 2020 Apr 17. FASEB J. 2020. PMID: 32301551
-
The effect of mechanical stimulation on the maturation of TDSCs-poly(L-lactide-co-e-caprolactone)/collagen scaffold constructs for tendon tissue engineering.Biomaterials. 2014 Mar;35(9):2760-72. doi: 10.1016/j.biomaterials.2013.12.042. Epub 2014 Jan 8. Biomaterials. 2014. PMID: 24411676
-
Repair of tendon defect with dermal fibroblast engineered tendon in a porcine model.Tissue Eng. 2006 Apr;12(4):775-88. doi: 10.1089/ten.2006.12.775. Tissue Eng. 2006. PMID: 16674291
-
Tendon tissue engineering: biomechanical considerations.Biomed Mater. 2020 Jul 16;15(5):052001. doi: 10.1088/1748-605X/ab852f. Biomed Mater. 2020. PMID: 32235051 Review.
-
Mechanical Actuation Systems for the Phenotype Commitment of Stem Cell-Based Tendon and Ligament Tissue Substitutes.Stem Cell Rev Rep. 2016 Apr;12(2):189-201. doi: 10.1007/s12015-015-9640-6. Stem Cell Rev Rep. 2016. PMID: 26661573 Review.
Cited by
-
Hyperuricemic PRP in tendon cells.Biomed Res Int. 2014;2014:926481. doi: 10.1155/2014/926481. Epub 2014 Sep 8. Biomed Res Int. 2014. PMID: 25276832 Free PMC article.
-
State-of the-art and future perspective in co-culture systems for tendon engineering.Biomater Biosyst. 2025 Mar 5;17:100110. doi: 10.1016/j.bbiosy.2025.100110. eCollection 2025 Mar. Biomater Biosyst. 2025. PMID: 40130022 Free PMC article.
-
Macrophage Mechano-Responsiveness Within Three-Dimensional Tissue Matrix upon Mechanotherapy-Associated Strains.Tissue Eng Part A. 2024 Apr;30(7-8):314-329. doi: 10.1089/ten.TEA.2023.0110. Epub 2023 Oct 27. Tissue Eng Part A. 2024. PMID: 37725574 Free PMC article.
-
Biglycan expression in the melanoma microenvironment promotes invasiveness via increased tissue stiffness inducing integrin-β1 expression.Oncotarget. 2017 Jun 27;8(26):42901-42916. doi: 10.18632/oncotarget.17160. Oncotarget. 2017. PMID: 28476030 Free PMC article.
-
Cellular homeostatic tension and force transmission measured in human engineered tendon.J Biomech. 2018 Sep 10;78:161-165. doi: 10.1016/j.jbiomech.2018.07.032. Epub 2018 Jul 30. J Biomech. 2018. PMID: 30100218 Free PMC article.
References
-
- Giddings VL, Beaupre GS, Whalen RT, Carter DR (2000) Calcaneal loading during walking and running. Med Sci Sports Exerc 32: 627–634. - PubMed
-
- Herchenhan A, Bayer ML, Svensson RB, Magnusson SP, Kjaer M (2013) In vitro tendon tissue development from human fibroblasts demonstrates collagen fibril diameter growth associated with a rise in mechanical strength. Dev Dyn 242: 2–8. - PubMed
-
- Couppe C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, et al. (2008) Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol 105: 805–810. - PubMed
-
- Eliasson P, Andersson T, Aspenberg P (2012) Achilles tendon healing in rats is improved by intermittent mechanical loading during the inflammatory phase. J Orthop Res 30: 274–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

