Alteration of daily and circadian rhythms following dopamine depletion in MPTP treated non-human primates
- PMID: 24465981
- PMCID: PMC3900505
- DOI: 10.1371/journal.pone.0086240
Alteration of daily and circadian rhythms following dopamine depletion in MPTP treated non-human primates
Abstract
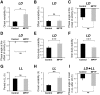
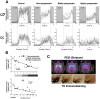
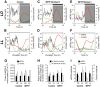
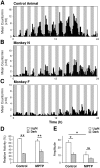
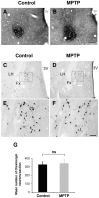
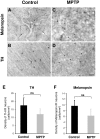
Disturbances of the daily sleep/wake cycle are common non-motor symptoms of Parkinson's disease (PD). However, the impact of dopamine (DA) depletion on circadian rhythms in PD patients or non-human primate (NHP) models of the disorder have not been investigated. We evaluated alterations of circadian rhythms in NHP following MPTP lesion of the dopaminergic nigro-striatal system. DA degeneration was assessed by in vivo PET ([(11)C]-PE2I) and post-mortem TH and DAT quantification. In a light∶dark cycle, control and MPTP-treated NHP both exhibit rest-wake locomotor rhythms, although DA-depleted NHP show reduced amplitude, decreased stability and increased fragmentation. In all animals, 6-sulphatoxymelatonin peaks at night and cortisol in early morning. When the circadian system is challenged by exposure to constant light, controls retain locomotor rest-wake and hormonal rhythms that free-run with stable phase relationships whereas in the DA-depleted NHP, locomotor rhythms are severely disturbed or completely abolished. The amplitude and phase relations of hormonal rhythms nevertheless remain unaltered. Use of a light-dark masking paradigm shows that expression of daily rest-wake activity in MPTP monkeys requires the stimulatory and inhibitory effects of light and darkness. These results suggest that following DA lesion, the central clock in the SCN remains intact but, in the absence of environmental timing cues, is unable to drive downstream rhythmic processes of striatal clock gene and dopaminergic functions that control locomotor output. These findings suggest that the circadian component of the sleep-wake disturbances in PD is more profoundly affected than previously assumed.
Conflict of interest statement
Figures


Similar articles
-
Loss of dopamine disrupts circadian rhythms in a mouse model of Parkinson's disease.Neurobiol Dis. 2014 Nov;71:359-69. doi: 10.1016/j.nbd.2014.08.024. Epub 2014 Aug 27. Neurobiol Dis. 2014. PMID: 25171792
-
Lack of long-term changes in circadian, locomotor, and cognitive functions in acute and chronic MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse models of Parkinson's disease.Chronobiol Int. 2013 Jul;30(6):741-55. doi: 10.3109/07420528.2012.762011. Epub 2013 Jun 12. Chronobiol Int. 2013. PMID: 23758587
-
Circadian and dark-pulse activation of orexin/hypocretin neurons.Mol Brain. 2008 Dec 3;1:19. doi: 10.1186/1756-6606-1-19. Mol Brain. 2008. PMID: 19055781 Free PMC article.
-
alpha-Synuclein- and MPTP-generated rodent models of Parkinson's disease and the study of extracellular striatal dopamine dynamics: a microdialysis approach.CNS Neurol Disord Drug Targets. 2010 Aug;9(4):482-90. doi: 10.2174/187152710791556177. CNS Neurol Disord Drug Targets. 2010. PMID: 20522009 Review.
-
The MPTP-treated primate as a model of motor complications in PD: primate model of motor complications.Neurology. 2003 Sep 23;61(6 Suppl 3):S4-11. doi: 10.1212/wnl.61.6_suppl_3.s4. Neurology. 2003. PMID: 14504374 Review.
Cited by
-
Modeling Parkinson's Disease: Not Only Rodents?Front Aging Neurosci. 2021 Aug 6;13:695718. doi: 10.3389/fnagi.2021.695718. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34421573 Free PMC article.
-
Circadian disruption and human health.J Clin Invest. 2021 Oct 1;131(19):e148286. doi: 10.1172/JCI148286. J Clin Invest. 2021. PMID: 34596053 Free PMC article. Review.
-
Motivational and Valence-Related Modulation of Sleep/Wake Behavior are Mediated by Midbrain Dopamine and Uncoupled from the Homeostatic and Circadian Processes.Adv Sci (Weinh). 2022 Aug;9(24):e2200640. doi: 10.1002/advs.202200640. Epub 2022 Jul 6. Adv Sci (Weinh). 2022. PMID: 35794435 Free PMC article.
-
Deep Brain Stimulation and Sleep-Wake Disturbances in Parkinson Disease: A Review.Front Neurol. 2018 Aug 27;9:697. doi: 10.3389/fneur.2018.00697. eCollection 2018. Front Neurol. 2018. PMID: 30210429 Free PMC article. Review.
-
Abrogation of the Circadian Nuclear Receptor REV-ERBα Exacerbates 6-Hydroxydopamine-Induced Dopaminergic Neurodegeneration.Mol Cells. 2018 Aug 31;41(8):742-752. doi: 10.14348/molcells.2018.0201. Epub 2018 Jul 30. Mol Cells. 2018. PMID: 30078232 Free PMC article.
References
-
- Chaudhuri KR, Healy DG, Schapira AH (2006) Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 5: 235–245. - PubMed
-
- Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8: 464–474. - PubMed
-
- Carlsson A (1972) Biochemical and pharmacological aspects of Parkinsonism. Acta Neurol Scand Suppl 51: 11–42. - PubMed
-
- Arnulf I, Leu-Semenescu S (2009) Sleepiness in Parkinson's disease. Parkinsonism Relat Disord 15: S101–S104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

